Abstract
Background
Changes in the composition of gastrointestinal microbiota by dietary interventions using pro- and prebiotics provide opportunity for improving health and preventing disease. However, the capacity of lupin kernel fiber (LKFibre), a novel legume-derived food ingredient, to act as a prebiotic and modulate the colonic microbiota in humans needed investigation.
Aim of the study
The present study aimed to determine the effect of LKFibre on human intestinal microbiota by quantitative fluorescent in situ hybridization (FISH) analysis.
Design
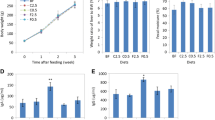
A total of 18 free-living healthy males between the ages of 24 and 64 years consumed a control diet and a LKFibre diet (containing an additional 17–30 g/day fiber beyond that of the control—incorporated into daily food items) for 28 days with a 28-day washout period in a single-blind, randomized, crossover dietary intervention design.
Methods
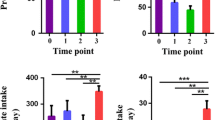
Fecal samples were collected for 3 days towards the end of each diet and microbial populations analyzed by FISH analysis using 16S rRNA gene-based oligonucleotide probes targeting total and predominant microbial populations.
Results
Significantly higher levels of Bifidobacterium spp. (P = 0.001) and significantly lower levels of the clostridia group of C. ramosum, C. spiroforme and C. cocleatum (P = 0.039) were observed on the LKFibre diet compared with the control. No significant differences between the LKFibre and the control diet were observed for total bacteria, Lactobacillus spp., the Eubacterium spp., the C. histolyticum/C. lituseburense group and the Bacteroides–Prevotella group.
Conclusions
Ingestion of LKFibre stimulated colonic bifidobacteria growth, which suggests that this dietary fiber may be considered as a prebiotic and may beneficially contribute to colon health.
Similar content being viewed by others
References
Kolida S, Tuohy K, Gibson GR (2000) The human gut flora in nutrition and approaches for its dietary modulation. Nutr Bull 25:223–231
Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB (2004) Dietary modulation of the human colonic microbiota: updating the concept of probiotics. Nutr Res Rev 17:259–275
Collins MD, Gibson GR (1999) Probiotics, prebiotics and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69: 1052S–1057S
Rao VA (2001) The prebiotic properties of oligofructose at low intake levels. Nutr Res 21:843–848
Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW (2000) Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 30:61–67
Tuohy KM, Finlay RK, Wynne AG, Gibson GR (2001a) A human volunteer study on the prebiotic effects of HP-inulin-faecal bacteria enumerated using fluorescent in situ hybridisation (FISH). Anaerobe 7:113–118
Harmsen HJM, Raangs GC, Franks AH, Wildeboer-Veloo ACM, Welling GW (2003) The effect of the prebiotic inulin and the probiotic Bifidobacterium longum on the faecal microbiota of healthy volunteers measured by FISH and DGGE. Microb Ecol Health Dis 14:211–219
McCracken VJ, Simpson JM, Mackie RI, Gaskins HR (2001) Molecular ecological analysis of the dietary and antibiotic-induced alterations of the mouse intestinal microbiota. J Nutr 131:1862–1870
Clark RL, Johnson SJ (2002) Sensory acceptability of foods with added lupin (Lupinus angustifolius) kernel fiber using pre-set criteria. J Food Sci 67:356–362
Hall RS, Johnson SK, Baxter AL, Ball MJ (2005) Lupin kernel fiber-enriched foods beneficially modify serum lipids in men. Eur J Clin Nutr 59:325–333
Evans AJ, Cheung PCK (1993) The carbohydrate composition of cotyledons and hulls of cultivars of Lupinus angustifolius from Western Australia. J Sci Food Agric 61:189–194
Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW (1998) Variations of bacterial populations in human feces measured by fluorescent in situ hybridisation with group-specific 16S rRNA oligonucleotide probes. Appl Environ Microbiol 64:3336–3345
Collins MD, Lawson PA, Willems A, Cordona JJ, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow JA (1994) The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol 44:812–826
Poulsen LKL, Lan F, Kristensen CS, Hobolth P, Molin S, Krogfelt KA (1994) Spatial distribution of Escherichia coli in the mouse large intestine inferred from rRNA in situ hybridisation. Infect Immun 62:5191–5194
Harmsen HJM, Raangs GC, He T, Degener JE, Welling GW (2002) Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl Environ Microbiol 68:2982–2990
Wildeboer-Veloo ACM, Harmsen HJM, Degener JE, Welling GW (2003) Development of a 16S rRNA-based probe for Clostridum ramosum, C. spiroforme and C. cocleatum and its application for the quantification in human faeces from volunteers of different age groups. Microb Ecol Health Dis 15:131–136
Gibson GR, Beatty ER, Wang X, Cummings JH (1995) Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterol 108:975–982
Pathmakanthan S, Thornley JP, Hawkey CJ (1999) Mucosally associated bacterial flora of the human colon: quantitative and species specific differences between normal and inflamed colonic biopsies. Microbial Ecol Health Dis 11:169–174
Gibson GR, Wang X (1994) Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol 77:412–420
Langlands SJ, Hopkins MJ, Coleman N, Cummings JH (2005) Prebiotic carbohydrates modify the mucosa associated microbiota of the human large bowel. Gut 53:1610–1616
Rubio LA, Spencer R, Grant G, Pustzai A (1995) The inclusion of lupin (Lupines angustifolius) seed meal or its fiber residue in the diet reduces the levels of Escherichia coli in both small and large intestines of the rat. Microb Ecol Health Dis 8:101–105
Zoetendal EG, Akkermans ADL, Akkermans van-Vliet WM, de Visser JAGM, de Vos WM (2001) The host genotype affects the bacterial community in the human gastrointestinal tract. Microbial Ecol Health Dis 13:129–134
Tuohy KM, Kolida S, Lustenberger AM, Gibson GR (2001) The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oliogosaccharides—a human volunteer study. Br J Nutr 86:341–348
Johnson SK, Chua V, Hall RS, Baxter AL (2006) Lupin kernel fiber foods improve bowel function and beneficially modify some putative faecal risk factors for colon cancer in men. Br J Nutr 95:372–378
Gois-Rigottier L, Rochet V, Garrec N, Suau A, Dore J (2003) Enumeration of bacteroides species in human faeces by fluorescent in situ hybridisation combined with flow cytometry using 16S rRNA probes. Syst Appl Micr 26:110–118
Acknowledgements
The dietary study was supported by the Grains Research and Development Corporation, the Australian Research Council—Strategic Partnerships with Industry—Research and Training Scheme, the Department of Agriculture Western Australia and Deakin University. The authors are grateful to Food Science Australia for manufacturing the LKFibre, to George Weston Foods for manufacturing the experimental food products and conducting nutritional analyses of these foods and to the study participants for their commitment to the study protocol. The authors also express their thanks to M. Ball for advice on the dietary design, Ms Amynta Baxter and Ms Veronica Chua for their assistance with some parts of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, S.C., Choy, R., Johnson, S.K. et al. Lupin kernel fiber consumption modifies fecal microbiota in healthy men as determined by rRNA gene fluorescent in situ hybridization. Eur J Nutr 45, 335–341 (2006). https://doi.org/10.1007/s00394-006-0603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-006-0603-1