Abstract
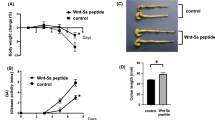
Previously we demonstrated an ameliorating effect of the interleukin-1ß converting enzyme (ICE) inhibitor pralnacasan on dextran sulfate sodium (DSS)-induced colitis. This study investigates the effects of pralnacasan on cytokine expression in DSS-induced colitis. Colitis was induced by oral administration of DSS. Mice were treated intraperitoneally with the ICE inhibitor pralnacasan (50 mg/kg body weight twice daily). Body weight as well as the presence of occult blood or diarrhea was monitored daily. Subgroups were sacrificed at days 4, 8, and 11 after the beginning of DSS application. Cytokine profiles in colonic tissue were analyzed on the protein level by ELISA and on the mRNA level by real time RT-PCR. Administration of DSS led to an increase in IL-18, IL-12, TNF-α, and IFN-γ protein as well as IP-10 and TNF-α mRNA. The increase in IL-18 and IFN-γ was reduced by ICE inhibition. Pralnacasan prevented DSS-induced colitis in C57BL/6 mice. In C57BL/6 mice, the DSS-induced increase in IP-10 mRNA, but not TNF-α mRNA, was completely prevented by ICE inhibition. In conclusion, prevention of colitis in C57BL/6 mice was associated with a suppresion of IP-10 mRNA, but not TNF-α mRNA expression, indicating that IL-18-mediated cytokine production is a key element in the pathogenesis of DSS-induced colitis.







Similar content being viewed by others
References
MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A (1990) Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 81(2):301–305
Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F (1999) IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease:expression and localization in intestinal mucosal cells. J Immunol 162(11):6829–6835
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353(23):2462–2476
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH (1995) Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 102(3):448–455
Panes J (2001) Inflammatory bowel disease:pathogenesis and targets for therapeutic interventions. Acta Physiol Scand 173(1):159–165
Wyatt J, Oberhuber G, Pongratz S, Puspok A, Moser G, Novacek G, Lochs H, Vogelsang H (1997) Increased gastric and intestinal permeability in patients with Crohn's disease. Am J Gastroenterol 92(10):1891–1896
Stokkers PC, Hommes DW (2004) New cytokine therapeutics for inflammatory bowel disease. Cytokine 28(4–5):167–173
Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ (1999) Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 340(18):1398–1405
Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ (1997) A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 337(15):1029–1035
Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M (1996) Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells:synergism with interleukin-12 for interferon-gamma production. Eur J Immunol 26(7):1647–1651
Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, Drillenburg P, van Deventer SJ, Chvatchko Y, Te Velde AA (2001) Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology 121(6):1372–1379
Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, Hartmann G, Dinarello CA, Endres S, Eigler A (2001) Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol 281(4):R1264–R1273
Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, Olson A, Bao W, Rutgeerts P (2004) Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol 2(7):542–553
Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OO, Innes A (2005) A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology 129(3):807–818
Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P (2006) Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology 130(2):323–333, quiz 591
Fantuzzi G, Reed DA, Dinarello CA (1999) IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest 104(6):761–767
Nadiri A, Wolinski MK, Saleh M (2006) The inflammatory caspases:key players in the host response to pathogenic invasion and sepsis. J Immunol 177(7):4239–4245
Watson RW, Rotstein OD, Parodo J, Bitar R, Marshall JC (1998) The IL-1 beta-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1 beta. J Immunol 161(2):957–962
Siegmund B (2002) Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol 64(1):1–8
Ku G, Ford BS, Raybuck SA, Harding MW, Randle JCR (2001) Selective interleukin-1 converting enzyme (ICE/caspase-1) inhibition with pralnacasan (HMR 3480/VX-740) reduces inflammation and joint destruction in murine type II collagen-induced arthritis (CIA). Am Coll Rheumatol 12–15 Nov:Abs 1134
Rudolphi K, Gerwin N, Verzijl N, Van Der Kraan P, Van Den Berg W (2003) Pralnacasan, an inhibitor of interleukin-1beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis Cartilage 11(10):738–746
Vertex Pharmaceuticals Inc. (2002) Phase IIa clinical trial results for pralnacasan in rheumatoid arthritis, presented at the 66th Annual Scientific Meeting of the American College of Rheumatology (ACR), New Orleans, October 24–29 (press release); available at: http://www.vpharm.com/Pressreleases2002/pr102902.html
Loher F, Bauer C, Landauer N, Schmall K, Siegmund B, Lehr HA, Dauer M, Schoenharting M, Endres S, Eigler A (2004) The interleukin-1 beta-converting enzyme inhibitor pralnacasan reduces dextran sulfate sodium-induced murine colitis and T helper 1 T-cell activation. J Pharmacol Exp Ther 308(2):583– 590
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98(3):694–702
Autenrieth IB, Bucheler N, Bohn E, Heinze G, Horak I (1997) Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with bowel inflammation. Gut 41(6):793–800
Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M (1999) Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol 162(3):1685–1691
Stevceva L, Pavli P, Husband A, Ramsay A, Doe WF (2001) Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes Immun 2(6):309–316
Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69(2):238–249
Venkatraman A, Ramakrishna BS, Pulimood AB, Patra S, Murthy S (2000) Increased permeability in dextran sulphate colitis in rats: time course of development and effect of butyrate. Scand J Gastroenterol 35(10):1053–1059
Kitajima S, Takuma S, Morimoto M (2000) Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim 49(1):9–15
Sasaki T, Horiuchi S, Yamazaki M, Yui S (1996) Stimulation of macrophage DNA synthesis by polyanionic substances through binding to the macrophage scavenger receptor. Biol Pharm Bull 19(3):449–455
Araki Y, Andoh A, Fujiyama Y, Bamba T (2000) Development of dextran sulphate sodium-induced experimental colitis is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin Exp Immunol 119(2):264–269
Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO (1994) Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107(6):1643–1652
Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC (1996) Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res 45(4):181–191
Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T (1998) Involvement of CD4+ T cells in the development of dextran sulfate sodium-induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol 31(3):477–481
Charles PC, Weber KS, Cipriani B, Brosnan CF (1999) Cytokine, chemokine and chemokine receptor mRNA expression in different strains of normal mice: implications for establishment of a Th1/Th2 bias. J Neuroimmunol 100(1–2):64–73
Ritchie AJ, Yam AO, Tanabe KM, Rice SA, Cooley MA (2003) Modification of in vivo and in vitro T- and B-cell-mediated immune responses by the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun 71(8):4421–4431
Wells CA, Ravasi T, Faulkner GJ, Carninci P, Okazaki Y, Hayashizaki Y, Sweet M, Wainwright BJ, Hume DA (2003) Genetic control of the innate immune response. BMC Immunol 4(1):5
Tomoyose M, Mitsuyama K, Ishida H, Toyonaga A, Tanikawa K (1998) Role of interleukin-10 in a murine model of dextran sulfate sodium-induced colitis. Scand J Gastroenterol 33(4):435–440
Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL (2002) Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut 50(6):812–820
Loher F, Schmall K, Freytag P, Landauer N, Hallwachs R, Bauer C, Siegmund B, Rieder F, Lehr HA, Dauer M, Kapp JF, Endres S, Eigler A (2003) The specific type-4 phosphodiesterase inhibitor mesopram alleviates experimental colitis in mice. J Pharmacol Exp Ther 305(2):549–556
Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M (1999) Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol 155(2):331–336
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is part of the doctoral thesis of Christian Bauer.
Rights and permissions
About this article
Cite this article
Bauer, C., Loher, F., Dauer, M. et al. The ICE Inhibitor Pralnacasan Prevents DSS-Induced Colitis in C57BL/6 Mice and Suppresses IP-10 mRNA but Not TNF-α mRNA Expression. Dig Dis Sci 52, 1642–1652 (2007). https://doi.org/10.1007/s10620-007-9802-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9802-8