Abstract
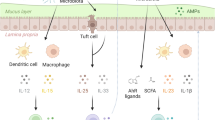
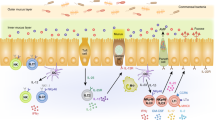
The mucosal immune system of the intestine is separated from a vast array of microbes by a single layer of epithelial cells. Cues from the commensal microflora are needed to maintain epithelial homeostasis, but the molecular and cellular identities of these cues are unclear. Here we provide evidence that signals from the commensal microflora contribute to the differentiation of a lymphocyte population coexpressing stimulatory natural killer cell receptors and the transcription factor RORγt that produced interleukin 22 (IL-22). The emergence of these IL-22-producing RORγthiNKp46+NK1.1int cells depended on RORγt expression, which indicated that these cells may have been derived from lymphoid tissue–inducer cells. IL-22 released by these cells promoted the production of antimicrobial molecules important in the maintenance of mucosal homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Newberry, R.D. & Lorenz, R.G. Organizing a mucosal defense. Immunol. Rev. 206, 6–21 (2005).
Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8, 411–420 (2008).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Cash, H.L., Whitham, C.V., Behrendt, C.L. & Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Kanamori, Y. et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+IL-7R+Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 184, 1449–1459 (1996).
Eberl, G. & Littman, D.R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Kurebayashi, S. et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. USA 97, 10132–10137 (2000).
Eberl, G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat. Rev. Immunol. 5, 413–420 (2005).
Niess, J.H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Tagliabue, A., Befus, A.D., Clark, D.A. & Bienenstock, J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J. Exp. Med. 155, 1785–1796 (1982).
Hogan, P.G., Hapel, A.J. & Doe, W.F. Lymphokine-activated and natural killer cell activity in human intestinal mucosa. J. Immunol. 135, 1731–1738 (1985).
Gibson, P.R. & Jewell, D.P. The nature of the natural killer (NK) cell of human intestinal mucosa and mesenteric lymph node. Clin. Exp. Immunol. 61, 160–168 (1985).
Dumoutier, L., Louahed, J. & Renauld, J.C. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 164, 1814–1819 (2000).
Dumoutier, L., Van Roost, E., Colau, D. & Renauld, J.C. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl. Acad. Sci. USA 97, 10144–10149 (2000).
Xie, M.H. et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2–4 and IL-22R. J. Biol. Chem. 275, 31335–31339 (2000).
Liang, S.C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Karin, M., Lawrence, T. & Nizet, V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124, 823–835 (2006).
Walzer, T. et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA 104, 3384–3389 (2007).
Sivori, S. et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J. Exp. Med. 186, 1129–1136 (1997).
Di Santo, J.P. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 24, 257–286 (2006).
Arase, H., Saito, T., Phillips, J.H. & Lanier, L.L. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (α2 integrin, very late antigen-2). J. Immunol. 167, 1141–1144 (2001).
Kim, S. et al. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 3, 523–528 (2002).
Hayakawa, Y. & Smyth, M.J. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176, 1517–1524 (2006).
DiSanto, J.P., Müller, W., Guy-Grand, D., Fischer, A. & Rajewsky, K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA 92, 377–381 (1995).
Kennedy, M.K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Saito, H. et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science 280, 275–278 (1998).
Suzuki, K. et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 13, 691–702 (2000).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Mebius, R.E., Rennert, P. & Weissman, I.L. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Graf, R. et al. Exocrine meets endocrine: pancreatic stone protein and regenerating protein–two sides of the same coin. J. Surg. Res. 133, 113–120 (2006).
Cupedo, T. et al. Human fetal lymphoid tissue–inducer cells are interleukin 17–producing precursors to RORC+ CD127+ natural killer cells. Nat. Immunol. advance online publication, doi:10.1038/ni1668 (23 November 2008).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. advance online publication, doi:10.1038/ni1681 (23 November 2008).
Lucas, M., Schachterle, W., Oberle, K., Aichele, P. & Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26, 503–517 (2007).
Diefenbach, A. & Raulet, D.H. Innate immune recognition by stimulatory immunoreceptors. Curr. Opin. Immunol. 15, 37–44 (2003).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Lotz, M. et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203, 973–984 (2006).
Acknowledgements
We thank M. Kist for support; M. Lucas, H. Pircher, W. Schachterle and C. Vonarbourg for critical comments on the manuscript; M. Schnare for discussions; K. Geiger and M. Follo for cell sorting; D. Littman (New York University) for Rorc(γt)GFP/+ mice; B. Stockinger (National Institute for Medical Research Mill Hill) for support and reagents; M. Kopf (Swiss Federal Institute of Technology Zürich) for Il6−/− mice on a B6 background; J.-C. Renauld (Ludwig Institute for Cancer Research Brussels) for anti-IL-22; and N. Goeppert for technical assistance. Supported by Deutsche Forschungsgemeinschaft (Di 764/2-2, GRK1104 (A.M.) and SFB620).
Author information
Authors and Affiliations
Contributions
S.L.S., V.L.B., A.M., K.O. and C.H. did and analyzed the experiments; C.J. generated and provided the germ-free mice and contributed to the experimental design; and A.D. and S.L.S. designed the experiments and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Methods (PDF 113 kb)
Rights and permissions
About this article
Cite this article
Sanos, S., Bui, V., Mortha, A. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nat Immunol 10, 83–91 (2009). https://doi.org/10.1038/ni.1684
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1684
This article is cited by
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
BMC Medicine (2024)
-
The emerging family of RORγt+ antigen-presenting cells
Nature Reviews Immunology (2024)
-
Proline uptake promotes activation of lymphoid tissue inducer cells to maintain gut homeostasis
Nature Metabolism (2023)
-
Identification of two migratory colon ILC2 populations differentially expressing IL-17A and IL-5/IL-13
Science China Life Sciences (2023)
-
The unique role of innate lymphoid cells in cancer and the hepatic microenvironment
Cellular & Molecular Immunology (2022)