Abstract
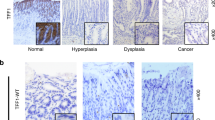
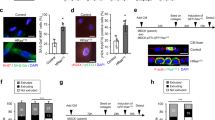
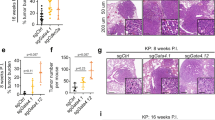
The latent transcription factor Stat3 is activated by gp130, the common receptor for the interleukin (IL)-6 cytokine family and other growth factor and cytokine receptors. Ligand-induced dimerization of gp130 leads to activation of the Stat1, Stat3 and Shp2-Ras-Erk signaling pathways. Here we assess genetically the contribution of exaggerated Stat3 activation to the phenotype of gp130Y757F/Y757F mice, in which a knock-in mutation disrupts the negative feedback mechanism on gp130-dependent Stat signaling. Compared to gp130Y757F/Y757F mice, reduced Stat3 activation in gp130Y757F/Y757FStat3+/− mice increased their lifespan, prevented splenomegaly, normalized exaggerated hepatic acute-phase response and lymphocyte trafficking, and suppressed the growth of spontaneously arising gastric adenomas in young mice. These lesions share histological features of gastric polyps in aging mice with monoallelic null mutations in Smad4, which encodes the common transducer for transforming growth factor (TGF)-β signaling. Indeed, hyperactivation of Stat3 desensitizes gp130Y757F/Y757F cells to the cytostatic effect of TGF-β through transcriptional induction of inhibitory Smad7, thereby providing a novel link for cross-talk between Stat and Smad signaling in gastric homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Maritano, D. et al. The STAT3 isoforms alpha and beta have unique and specific functions. Nat. Immunol. 5, 401–409 (2004).
Levy, D.E. & Lee, C.K. What does Stat3 do? J. Clin. Invest. 109, 1143–1148 (2002).
Tebbutt, N.C. et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 8, 1089–1097 (2002).
Heinrich, P.C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Croker, B.A. et al. SOCS3 negatively regulates IL-6 signaling in vivo . Nat. Immunol. 4, 540–545 (2003).
Lefebvre, O. et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 274, 259–262 (1996).
Shi, Y. & Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Nakao, A. et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 389, 631–635 (1997).
Massague, J., Blain, S.W. & Lo, R.S. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103, 295–309 (2000).
Boivin, G.P. et al. Gastric lesions in transforming growth factor beta-1 heterozygous mice. Lab. Invest. 74, 513–518 (1996).
Takaku, K. et al. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 59, 6113–6117 (1999).
Xu, X. et al. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 19, 1868–1874 (2000).
Hohenstein, P. et al. Serrated adenomas and mixed polyposis caused by a splice acceptor deletion in the mouse Smad4 gene. Genes Chromosom. Cancer 36, 273–282 (2003).
Jenkins, B.J., Roberts, A.W., Najdovska, M., Grail, D. & Ernst, M. The threshold of gp130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood 105, 3512–3520 (2005).
Takeda, K. et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA 94, 3801–3804 (1997).
Judd, L.M. et al. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 126, 196–207 (2004).
Hurst, S.M. et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14, 705–714 (2001).
Peters, M. et al. Extramedullary expansion of hematopoietic progenitor cells in interleukin (IL)-6-sIL-6R double transgenic mice. J. Exp. Med. 185, 755–766 (1997).
Ernst, M. & Jenkins, B.J. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 20, 23–32 (2004).
Bromberg, J.F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999).
Fischer, M. et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat. Biotechnol. 15, 142–145 (1997).
Ulloa, L., Doody, J. & Massague, J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature 397, 710–713 (1999).
Zhu, H.-J., Iaria, J. & Sizeland, A.M. Smad7 differentially regulates transforming growth factor-β mediated signaling pathways. J. Biol. Chem. 274, 32258–32264 (1999).
Kanai, M. et al. Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK-ERK-dependent pathway. Oncogene 22, 548–554 (2003).
Leong, P.L. et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc. Natl. Acad. Sci. USA 100, 4138–4143 (2003).
Kanda, N. et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene 23, 4921–4929 (2004).
Lauren, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histological classification. Acta Pathol. Microbiol. Scand. 64, 31–49 (1965).
Boussioutas, A. et al. Distinctive patterns of gene expression in premalignant gastric mucosa and gastric cancer. Cancer Res. 63, 2569–2577 (2003).
Gong, W. et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin. Cancer Res. 11, 1386–1393 (2005).
Papadimitrakopoulou, V.A. et al. Presence of multiple incontiguous deleted regions at the long arm of chromosome 18 in head and neck cancer. Clin. Cancer Res. 4, 539–544 (1998).
Xie, W. et al. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res. 62, 497–505 (2002).
Horvath, L.G. et al. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate 59, 234–242 (2004).
Li, Q.L. et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109, 113–124 (2002).
Brenner, O. et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc. Natl. Acad. Sci. USA 101, 16016–16021 (2004).
Becker, C. et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004).
Monteleone, G. et al. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J. Clin. Invest. 108, 601–609 (2001).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo . Nat. Med. 6, 583–588 (2000).
Howlett, M. et al. Differential regulation of gastric tumor growth by cytokines that signal exclusively through the co-receptor gp130. Gastroenterology (in the press).
Bossenmeyer-Pourie, C. et al. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J. Cell Biol. 157, 761–770 (2002).
Lerner, L., Henriksen, M.A., Zhang, X. & Darnell, J.E. Jr., STAT3-dependent enhanceosome assembly and disassembly: synergy with GR for full transcriptional increase of the alpha 2-macroglobulin gene. Genes Dev. 17, 2564–2577 (2003).
Darnell, J.E., Jr. Transcription factors as targets for cancer therapy. Nat. Rev. Cancer 2, 740–749 (2002).
Zhu, Y., Richardson, J.A., Parada, L.F. & Graff, J.M. Smad3 mutant mice develop metastatic colorectal cancer. Cell 94, 703–714 (1998).
Wang, Y. et al. Receptor subunit-specific action of oncostatin M in hepatic cells and its modulation by leukemia inhibitory factor. J. Biol. Chem. 275, 25273–25285 (2000).
Stenvers, K.L. et al. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol. Cell. Biol. 23, 4371–4385 (2003).
Brodin, G., Ahgren, A., ten Dijke, P., Heldin, C.H. & Heuchel, R. Efficient TGF-beta induction of the Smad7 gene requires cooperation between AP-1, Sp1, and Smad proteins on the mouse Smad7 promoter. J. Biol. Chem. 275, 29023–29030 (2000).
Jenkins, B.J. et al. Imbalanced gp130-dependent signaling in macrophages alters macrophage colony-stimulating factor responsiveness via regulation of c-fms expression. Mol. Cell. Biol. 24, 1453–1463 (2004).
Acknowledgements
We are indebted to S. Rose-John and G. Begley for the gift of HYPER-IL-6 and recombinant human IL-6, respectively. We thank A. Moustakas and R. Heuchel for making available the luciferase reporter constructs p(CAGA)12-luc and pSmad7-luc, and J. Wade for assistance in preparing plasma samples. We thank V. Feakes for histology, J. Stickland for photography and A. Burgess and J. Heath for critical reading of the manuscript. This work was supported by grants from the National Health and Medical Research Council of Australia to B.J.J., M.E., A.S.G. and H.J.Z., and by the Wellcome Trust to R.M.M.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Stat1 and Stat3 tyrosine phosphorylation in mouse embryonic fibroblasts (MEFs) stimulated with HYPER-IL-6. (PDF 109 kb)
Supplementary Fig. 2
Impaired TGF-β-dependent transcriptional activation of the artificial Smad3 reporter p(CAGA)12-luc in gastric epithelial IMGE-5 cells following activation of the gp130(Y757F) receptor. (PDF 85 kb)
Supplementary Table 1
Primers used in Real-time and Semi-quantitative RT-PCR assays. (PDF 74 kb)
Rights and permissions
About this article
Cite this article
Jenkins, B., Grail, D., Nheu, T. et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-β signaling. Nat Med 11, 845–852 (2005). https://doi.org/10.1038/nm1282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1282
This article is cited by
-
Loss of miR-26b-5p promotes gastric cancer progression via miR-26b-5p-PDE4B/CDK8-STAT3 feedback loop
Journal of Translational Medicine (2023)
-
Multiscale modeling of collective cell migration elucidates the mechanism underlying tumor–stromal interactions in different spatiotemporal scales
Scientific Reports (2022)
-
Nintedanib induces senolytic effect via STAT3 inhibition
Cell Death & Disease (2022)
-
Context-dependent functions of pattern recognition receptors in cancer
Nature Reviews Cancer (2022)
-
MSC secreted extracellular vesicles carrying TGF-beta upregulate Smad 6 expression and promote the regrowth of neurons in spinal cord injured rats
Stem Cell Reviews and Reports (2022)