Abstract
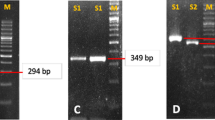
The existence ofhelicobacter pylori in the biliary tract was investigated. Seven bile samples were included in this study. Among them, six bile samples were collected by percutaneous transhepatic cholangiodrainage and the other by needle aspiration during cholecystectomy. Using nested PCR with two sets of primers homologous to the urease A gene,Helicobacter pylori DNA was detected. Three samples, one from a patient with advanced gastric cancer involving the pancreatic head and two from patients with pancreatic head tumor, were found to be positive forHelicobacter pylori DNA. On the other hand, three samples from patients with cholangiocarcinoma and one from a patient with chronic cholecystitis were all negative. To further verify the specificity of our PCR analysis, partial sequences of the PCR products from the three positive samples were analyzed by direct sequencing. Several silent mutations and a missense mutation (AAA to AGA; Lys-164 to Arg-164) were identified in the urease A gene. We conclude thatHelicobacter pylori DNA can be easily detected in the bile samples. The possibility of asymptomatic cholangitis caused by this organism requires further investigation.
Similar content being viewed by others
References
Graham DY:Helicobacter pylori: Its epidemiology and its role in duodenal ulcer disease. J Gastroenterol Hepatol 6:105–113, 1991
Graham DY, Malaty HM, Evans DG, Evans DJ Jr, Klein PD, Adam E: Epidemiology ofHelicobacter pylori in an asymptomatic population in the United States: Effect of age, race, and socioeconomic status. Gastroenterology 100:1496–1501, 1991
Cutler AF, Schubert TT: Patient factors affectingHelicobacter pylori eradication with triple therapy. Am J Gastroenterol 88:505–509, 1993
Laine L.: Antibiotic therapy alone eradicatesHelicobacter pylori and prevents duodenal ulcer recurrence. Gastroenterology 105:598–589, 1993
Vogt K, Hahn H: Urease production byHelicobacter pylori. Int J Med Microbiol 275:63–72, 1991
Katelaris PH, Lowe DG, Norbu P, Farthing MJ: Field evaluation of a rapid, simple and inexpensive urease test for the detection ofHelicobacter pylori. J Gastroenterol Hepatol 7:569–571, 1992
Loffeld RJ, Stobberingh E, Arends JW: A review of diagnostic techniques ofHelicobacter pylori infection. Dig Dis 11:173–180, 1993
Kolts BE, Joseph B, Achem SR, Bianchi T, Monteiro C:Helicobacter pylori detection: A quality and cost analysis. Am J Gastroenterol 88:650–655, 1993
Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S: Sensitive detection ofHelicobacter pylori by using polymerase chain reaction. J Clin Microbiol 30:192–200, 1992
Ho SA, Hoyle JA, Lewis FA, Secker AD, Cross D, Mapstone NP, Dixon MF, Wyatt JI, Tompkin DS, Taylor GR, Quirke P: Direct polymerase chain reaction test for detection ofHelicobacter pylori in humans and animals. J Clin Microbiol 29:2542–2549, 1991
Labigne A, Cssac V, Courcoux P: Shuttle cloning and nucleotide sequences ofHelicobacter pylori genes responsible for urease activity. J Bacteriol 173:1920–1931, 1991
Moore RA, Kureishi A, Wong S, Bryan LE: Categorization of clinical isolates ofHelicobacter pylori on the basis of restriction digest analyses of polymerase chain reaction-amplifiedureC genes. J Clin Microbiol 31:1334–1335, 1993
Mapstone NP, Lynch DAF, Lewis FA, Axon AT, Tompkins DS, Dixon MF: Identification ofHelicobacter pylori DNA in the mouths and stomachs of patients with gastritis using PCR. J Clin Pathol 46:540–543, 1993
Wesblom TU, Phadnis S, Yang P, Czinn ST: Diagnosis ofHelicobacter pylori infection by means of a polymerase chain reaction assay for gastric juice aspirates. Clin Infect Dis 16:367–371, 1993
Nguyen AM, Genta RM, Graham DY, Fouad AK: Detection ofHelicobacter pylori in dental plaque by reverse transcription polymerase chain reaction. J Clin Microbiol 31:783–787, 1993
Thomas JE, Gibson GR, Darboe MK, Dale A, Weaver LT: Isolation ofHelicobacter pylori from human feces. Lancet 340(8829):1194–1195, 1992
Varoli O, Landini MP, La Placa M, Tucci A, Corinaldesi R, Paparo GF, Stanghellini V, Barbara L: Presence ofHelicobacter pylori in gastric juice. Am J Gastroenterol 86:249, 1991
Prewett EJ, Bickley J, Owen RJ, Pounder RE: DNA pattern ofHelicobacter pylori isolated from gastric antrum, body and duodenum. Gastroenterology 102:829–833, 1992
Michael A, Lary MD, Donald E, Meier MD: Sphincter incompetence caused by common bile duct stones. Surgery 93:538–540, 1983
Floch MH, Gerhengoren W, Elliott S, Sp[ro HM: Bile acid inhibition of intestinal microflora. A function of simple bile acid? Gastroenterology 61:228–233, 1971
Williams RC, Showalter R, Kern F: The effect of bile salt and cholestyramine on intestinal anaerobic bacteria. Gastroenterology 69:483–491, 1975
Huhtanen CM: Bile acid inhibition ofClostridium botulinum. Appl Environ Microbiol 38:216–218, 1979
Hänninem ML: Sensitivity ofHelicobacter pylori to different bile salts. Eur J Clin Microl Infect Dis 10:515–518, 1991
West AP, Millard MR, Tompkins DS: Effect of physical environment on survival ofHelicobacter pylori. J Clin Pathol 45:228–231, 1992
Xu Guorong, Kirk CTC, Goode AW: Changes in biliary lipid concentrations in bile duct obstruction: An experimental study. J R Soc Med 79:522–527, 1986
Kellosalo J, Alavaikko M, Laitinen S: Effect of biliary tract produce on duodenogastric reflux and the gastric mucosa. Scand J Gastroenterol 26:1272–1278, 1991
Robles Campos R, Lujan Mompean JA, Parrilla Paricio P, Bermejo Lopez J, Liron Ruiz R, Torralba Martinez JA, Morales Cuenca G, Molina Martinzez JA: Role ofHelicobacter pylori infection and duodenogastric reflux in the pathogenesis of alkaline reflux gastritis after gastric operation. Surg Gynecol Obstet 176:594–598, 1993
Sobala GM, O'Connor HI, Dewar EP, King RF, Axon AT, Dixon MF: Bile reflux and intestinal metaplasia in gastric mucosa. J Clin Pathol 46:235–240, 1993
Author information
Authors and Affiliations
Additional information
This study was supported in part by grants from Chang Gung Medical Center (CMRPs) and also by a grant from the Prosperous Foundation, Taipei, Taiwan.
Rights and permissions
About this article
Cite this article
Lin, TT., Yeh, CT., Wu, CS. et al. Detection and partial sequence analysis ofHelicobacter pylori DNA in the bile samples. Digest Dis Sci 40, 2214–2219 (1995). https://doi.org/10.1007/BF02209009
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02209009