Abstract
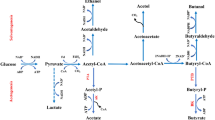
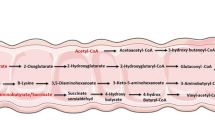
Butyrate is produced in the colon of mammals as a result of microbial fermentation of dietary fiber, undigested starch, and proteins. Butyrate may be an important protective agent in colonic carcinogenesis. Trophic effects on normal colonocytesin vitro andin vivo are induced by butyrate. In contrast, butyrate arrests the growth of neoplastic colonocytes and inhibits the preneoplastic hyperproliferation induced by some tumor promotersin vitro. We speculate that selective effects on G-protein activation may explain this paradox of butyrate's effects in normal versus neoplastic colonocytes. Butyrate induces differentiation of colon cancer cell lines. It also regulates the expression of molecules involved in colonocyte growth and adhesion and inhibits the expression of several protooncogenes relevant to colorectal carcinogenesis. Additional studies are needed to evaluate butyrate's antineoplastic effectsin vivo and to understand its mechanism(s) of action.
Similar content being viewed by others
References
Jacobs LR: Relationship between dietary fiber and cancer: metabolic, physiologic, and cellular mechanism. Proc Soc Exp Biol Med 182:299–310, 1986
Jacobs LR: Role of dietary factors in cell replication and colon cancer. Am J Clin Nutr 48:775–779, 1988
Jacobs LR: Influence of soluble fibers on experimental colon carcinogenesis.In Dietary Fiber: Basic and Clinical Aspects. GB Vahouny, D Kritchevesky (eds). New York, Plenum Press. 1990, pp 389–403
Lee DK, Chapkin RS, Lupton JR: Dietary fat and fiber modulate colonic cell proliferation in an interactive sitespecific manner. Nutr Cancer 20:107–118, 1993
Van Munster IP, Nagengast FM: The role of carbohydrate fermentation in colon cancer prevention. Scand J Gastroenterol 28(S200):80–86, 1993
McIntyre A, Gibson PR, Young GP: Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 34:386–391, 1993
Howe GR, Benito E, Castelleto R, Cornée J, Estève J, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R, Kune GA, Kune S. L'Abbé, Lee HP, Lee M, Miller AB, Peters RK, Potter JD, Riboli E, Slattery ML, Trichopoulos D, Tuyns A, Tzonou A, Whittemore AS, Wu-Williams AH, Shu Z: Dietary intake of fiber and decreased risk of cancers of the colon and rectum: Evidence from the combined analysis of 13 case-control studies. JNCI 84:1887–1896, 1992
Wolin MJ: Fermentation in the rumen and human large intestine. Science 213:1463–1468, 1981
Wrong OM: Carbohydrates.In The Large Intestine: Its Role in Mammalian Nutrition and Homeostasis. OM Wrong, CJ Edmonds, VS Chadwick (eds). New York, Halsted Press, 1981, pp 20–21, 113–114
Cummings JH, Branch WJ: Fermentation and production of short-chain fatty acids in human large intestine.In Dietary Fiber: Basic and Clinical Aspects. GB Vahouny and D Kritchevesky (eds). New York, Plenum Press, 1990, pp 131–152
Cummings JH, Pomare EW, Branch WJ, Naylor CPE, MacFarlane GT: Short chain fatty acids in human large intestine, portal, hepatic, and venous blood. Gut 28:1221–1227, 1987
Roediger WEW: Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83:424–429, 1982
Rombeau JL, Kripke SA, Settle RG: Short-chain fatty acids: Production, absorption, metabolism, and intestinal effects.In Dietary Fiber: Basic and Clinical Aspects. GB Vahouny and D Kritchevesky (eds) New York, Plenum Press, 1990, pp 317–339
Macfarlane GT, Gibson GR, Macfarlane S: Short-chain fatty acid and lactate production by human intestinal bacteria grown in batch and continuous culture.In Short-Chain Fatty Acids. J Cummings, HJ Binder, K Soergel (eds). Boston, Kluwer Academic Publishers, 1994, pp 44–60
Nyman M, Aso NG: Fermentation of dietary fiber components in rat intestinal tract. Br J Nutr 47:357–366, 1982
Demigné C, Remesy C: Stimulation of absorption of volatile fatty acids and minerals in cecum of rats adapted to a very high fiber diet. J Nutr 115:53–60, 1985
Rechkemmer G, Ronnau K, von Englehardt W: Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp Biochem Physiol 90A:563–568, 1988
Macfarlane GT, Gibson GR, Cummings JH: Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72:54–64, 1992
Titegeyer EC, Bourquin LD, Fahey GC, Garleb KA: Fermentability of various fiber sources by human fecal bacteriain vitro. Am J Clin Nutr 53:418–424, 1991
Knudsen KE, Jensen BB, Hansen I: Oat bran but not a β-glucan enriched oat fraction enhances butyrate production in the large intestine of pigs. J Nutr 123:1235–1247, 1993
Weaver GA, Krause JA, Miller TL, Wolin MJ: Cornstarch fermentation by the colonic microbial community yields more butyrate than does cabbage fiber fermentation: cornstarch fermentation rates correlate negatively with methanogenesis. Am J Clin Nutr 55:70–77, 1992
Colony PC: The identification of cell types in the normal adult colon.In Cell and Molecular Biology of Colon Cancer LH Augenlicht (ed). Boca Raton, CRC Press, 1989, pp 2–21
Higgins PJ: Antigenic and cytoarchitectural ‘markers’ of differentiation pathways in normal and malignant colonic epithelial cells.In Cell and Molecular Biology of Colon Cancer. LH Augenlicht (ed). Boca Raton, CRC Press, 1989, pp 112–132
Lipkin M, Enker WE, Winawer SJ: Tritiated-thymidine labeling of rectal epithelial cells in non-prep biopsics of individuals at increased risk for colonic neoplasia. Cancer Lett 37:153–161, 1987
Lipkin M: Biomarkers of increased susceptibility to gastrointestinal cancer: New application to studies of cancer prevention in human subjects. Cancer Res 48:235–245, 1988
Kim YS, Tsao D, Siddiqui B, Whitehead JS, Arnstein P, Bennette J, Hicks J: Effects of sodium butyrate and dimethylsulfoxide on biochemical properties of human colon cancer cells. Cancer 45:1185–1192, 1980
Tsao D, Morita A, Bella A, Luu P, Kim YS: Differential effects of sodium butyrate, dimethylfoxide, retinoic acid on membrane-associated antigen, enzymes and glycoproteins of human rectal adenocarcinoma cells. Cancer Res 42:1052–1058, 1982
Whitehead RH, Young GP, Bhathal PS: Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut 27:1427–1436, 1986
Czerniak B, Herz F, Westo RP, Koss LG: Modification of H-ras oncogene p-21 expression and cell cycle progression in the human colon cancer cell line HT-29. Cancer Res 47:2826–2830, 1987
Awad AB, Horvath PJ, Andersen MS: Influence of butyrate on lipid metabolism, survival, and differentiating of colon cancer cells. Nutr Cancer 16:125–133, 1991
Barnard JA, Warwick G: Sodium butyrate rapidly induces ‘enterocytic-like’ differentiation and growth inhibition of HT-29 cells. Gastroenterology 102:A199, 1992
Otaka M, Singhal A, Hakomori S: Antibody-mediated targeting of differentiation inducers to tumor cells: Inhibition of colonic cancer cell growthin vitro andin vivo. Biochem Biophys Res Commun 158:202–208, 1989
Velázquez OC, Jabbar A, DeMatteo RP, Rombeau JL: Butyrate inhibits hepatic tumor growth in a murine model of metastatic colorectal cancer. Surgery (in press).
Gibson PR, Moeller I, Kagelari O, Folino M, Young GP: Contrasting effects of butyrate on differentiation of neoplastic and nonneoplastic colonic epithelial cells. J Gastroenterol Hepatol 7:165–172, 1992
Sakata T: Influence of short-chain fatty acids on epithelial cell division of digestive tract. J Exp Physiol 69:639–648, 1984
Sakata T: Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fiber, gut microbes and luminal trophic factors. Br J Nutr 58:95–101, 1987
Kripke SA, Fox AD, Berman JM, Settle RG, Rombeau JL: Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. J Parenter Enter Nutr 13:109–116, 1989
Janne P, Carpenter Y, Willems G: Colonic mucosal atrophy induced by a liquid elemental diet in rats. Am J Dig Dis 22:808–812, 1977
Koruda MJ, Rolandelli RH, Bliss DZ, Hastings J, Rombeau JL, Settle RG: Parenteral nutrition supplemented with shortchain fatty acids: Effect on the small-bowel mucosa in normal rats. Am J Clin Nutr 51:685–689, 1990
Friedel D, Levine GM: Effect of short-chain fatty acids on colonic function and structure. J Parenter Enter Nutr 16(1):1–4, 1992
Lupton JR, Kurtz PP: Relationship of colonic luminal shortchain fatty acids and pH toin vivo cell proliferation in rats. J Nutr 123:1522–1530, 1993
Scheppach W, Bartram P, Richter A, Richter F, Dusel G, Liepold H, Hofstetter G, Ruthlein J, Kasper H: Effect of short-chain fatty acids on the human colonic mucosain vitro. J Parenter Enter Nutr 16:43–48, 1992
Sakata T, von Englehardt W: Stimulatory effects of short chain fatty acids on the epithelial cell proliferation in rat large intestine. Comp Biochem Physiol 74A:459–462, 1983
Frankel WL, Lew J, Su B, Bain A, Klurfeld D, Einhorn E, MacDermott RP, Rombeau JL: Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology 106:375–380, 1994
Sakata T: Stimulatory effect of short-chain fatty acids on epithelial cell proliferation of isolated and denervated jejunal segment of the rat. Scand J Gastroenterol 24:886–890, 1989
Toscani A, Soprano DR, Soprano KJ: Molecular analysis of sodium butyrate-induced growth arrest. Oncogene Res 3:223–238, 1988
Young GP, Gibson PR: Butyrate and colorectal cancer cell.In Short-Chain Fatty Acids. J Cummings, HJ Binder, K Soergel (eds). Boston, Kluwer Academic Publishers, 1994, pp 148–160
Barnard JA, Warwick G: Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ 4:495–501, 1993
Bartram HP, Scheppach W, Schmid H, Hofmann A, Dusel G, Richter F, Richter A, Kasper H: Proliferation of human colonic mucosa as an intermediate biomarker of carcinogenesis: Effects of butyrate, deoxycholate, calcium, ammonia, and pH. Cancer Res 53:3283–3288, 1993
Bartram HP, Scheppach W, Englert S, Dusel G, Richter A, Richter F, Kasper H: Effects of deoxycholic acid and butyrate on mucosal prostaglandin E2 release and cell proliferation in the human sigmoid colon. J Parenter Enter Nutr 19:182–186 1994
Velázquez OC, Zhou D, Seto R, Jabbar A, Choi J, Lederer HM, Rombeau J:In vivo crypt surface proliferation is decreased by butyrate and increased by deoxycholate in normal rat colon: Associatedin vivo effects on c-fos and c-jun expression. J Parenter Enter Nutr (in press)
Young GP, Gibson PR: Contrasting effects of butyrate on proliferation and differentiation of normal and neoplastic cells.In Short Chain Fatty Acids: Metabolism and Clinical Importance. Report of the Tenth Ross Conference on Medical Research. Columbus, Ohio, Ross Laboratories, 1991, pp 50–55
Young GP, Macrae FA, Gibson PR, Alexeyeff M, Whitehead R: Brush border hydrolases in normal and neoplastic colonic epithelium. J Gastroenterol Hepatol 7:347–357, 1992
Bell L, Williams L: Histochemical demonstration of alkaline phosphatase in human large intestine, normal and deceased. Histochemistry 60:84–90, 1979
Herz F, Schermer A, Halwer M, Bogart LH: Alkaline phosphatase in HT-29, a human colon cancer cell line: influence of sodium butyrate and hyperosmolality. Arch Biochem Biophys 210:581–591, 1981
Morita A, Tsao D, Kim YS: Effect of sodium butyrate on alkaline phosphatase in HRT-18, a human rectal cancer cell line. Cancer Res 42:4540–4545, 1982
Ito F, Chou JY: Induction of placental alkaline phosphatase biosynthesis by sodium butyrate. J Biol Chem 259:2526–2530, 1984
Chung YS, Song IS, Erickson RH, Sleisenger MH, Kim YS: Effect of growth and sodium butyrate on brush border membrane associated hydrolases in human colorectal cancer cell lines. Cancer Res 45:2976–2982, 1985
Hay FG, Duncan LW, Langdon SP, Leonard RCF: Modulation of the cluster 1 and mucin antigens in human small cell lung cancer and other epithelial tumour cell lines after treatment with the differentiation inducing agent, sodium butyrate. Br J Cancer 14:33–35, 1991
Frommel TO, Coon JS, Tsuro T, Roninson IB: Variable effects of sodium butyrate on the expression and function of the MDR-1 (P-glycoprotein) gene in colon carcinoma cell lines. Int J Cancer 55:297–302, 1993
Mickley LA, Batest SE, Richert ND, Currier S, Tanaka S, Foss F, Rosen N, Fojo AT: Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem 264:18031–18040, 1989
Petit JM, Chauffert B, Dimanche-Boitrel MT, Genne P, Duchamp O, Martin Francois: mdr 1 gene-expression and villin synthesis in a colon cancer cell line differentiated by sodium butyrate. Anticancer Res 13:487–490, 1993
White MW: Metabolism of the malignant cell,in vivo is anaerobic and significantly plays a factor in the pathway to carcinogenesis. Med Hypotheses 39(4):323–333 1992
Fischer H: Malignancy may contribute to rapid evolution. Med Hypotheses 28(1):35–38 1989
Isfort RJ, Cody DB, Asquith TN, Ridder GM, Stuard SB, LeBoeuf RA: Induction of protein phosphorylation, protein synthesis, immediate early gene expression and cellular proliferation by intracellular pH modulation. Eur J Biochem 213(1):349–357, 1993
Nathan DF, Burkhart SR, Morin MJ: Increased cell surface EGF receptor expression during the butyrate-induced differentiation of human HCT-116 colon tumor cell clone. Exp Cell Res 190:76–84, 1990
Kondoh N, Schweinfest CW, Henderson KW, Papas TS: Differential expression of s19 ribosomal protein, laminin-binding protein, and human lymphocyte antigen class 1 messenger RNAs associated with colon carcinoma progression and differentiation. Cancer Res 52:791–796, 1992
Boyd D, Florent G, Kim P, Brattain M: Determination of the levels of urokinase and its receptors in human colon carcinoma cell lines. Cancer Res 48:3112–3116, 1988
Kohga S, Harvey Sr, Weaver Rm, Markus G: Localization of plasminogen activators in human colon cancer by immunoperoxidase staining. Cancer Res 45:1787–1796, 1985
Gibson PR, Rosella O, Rosella G, Young GP: Butyrate is a potent inhibitor of urokinase secretion by normal colonic epitheliumin vitro. Gastroenterology 107(2):410–419, 1994
Bresalier RS, Rockwell RW, Dahiya R, Duh QY, Kim YS: Cell surface sialoprotein alterations in metastatic murine colon cancer cell lines selected in an animal model for colon cancer metastasis. Cancer Res 50:1299–1307, 1990
Bryant G, Haberern C, Rao CN, Liotta LA: Butyrate induced reduction of tumor cell laminin receptors. Cancer Res 46:807–811, 1986
Aumailley M, Nurcombe V, Edgar D, Paulsson M, Timpl R: The cellular interactions of laminin fragments. Cell adhesion correlates with two fragment-specific high affinity binding sites. J Biol Chem 262:11.532–11.538, 1987
Yannariello-Brown J, Weaver U, Liotta L, Madri JA: Distribution of a 69-kD laminin-binding protein in aortic and microvascular endothelial cell: Modulation during cell attachment, spreading, and migration. J Cell Biol 106:1773–1786, 1988
Herbst TJ, McCarthy JB, Tsilibary EC, Furcht LT: Differential effects of laminin, intact type IV collagen, and specific domains of type IV colagen on endothelial cell adhesion and migration. J Cell Biol 106:1365–1373, 1988
Ocalan M, Goodman SL, Kuhl U, Hanuschka SD, de Mark K: Laminin alters cell shape and stimulates motility and proliferation of marine skeletal myoblasts. Dev Biol 125:158–167, 1988
Kubota Y, Kleinman HK, Martin GR, Lawlwy TJ: Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 107:1589–1598, 1988
Dancker GW Jr, Piazza AJ, Steele GS, Jr, Mercurio AM: Relationship between extracellular matrix interactions and degree of differentiation in human colon carcinoma cell lines. Cancer Res 49:681–686, 1989
Terranova VP, Rao CN, Kalebie T, Margulies IM, Liotta LA: Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci USA 80:444–448, 1983
Wewer UM, Liotta LA, Jaye M, Ricca GA, Drohan WN, Claysmith AP, Rao CN, Wirth P, Coligan JE, Albrechtsen R, Mudryi M, Sobel ME: Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc Natl Acad Sci USA 83:7137–7141, 1986
Wilson JR, Weiser M: Colonic cancer cell (HT29) adhesion to laminin is altered by differentiation: Adhesion may involve galactosyltransferasc. Exp Cell Res 201:330–334, 1992
Fearon ER, Volgelstein B: A genetic model for colorectal tumorigenesis. Cell 61:759–767, 1990
Willson JKV: Biology of large bowel cancer. Hematol Oncol Clin North Am 3:19–34, 1989
Young GP, Gibson PR: Butyrate and the human cancer cell.In Physiologic and Clinical Aspects of Short-Chain Fatty Acids. JH Cummings, JL Rombeau, T Sakata (eds). Cambridge, Cambridge University Press, 1995, pp 319–336
Stoddart JH, Lane MA, Niles RM: Sodium butyrate suppresses the transforming activity of an activated N-ras oncogene in human colon carcinoma cells. Exp Cell Res 184:16–27, 1989
Foss FM, Veillette A, Sartor O, Rose N, Bolen JB: Alterations in the expression of pp60c-src and p56lck associated with butyrate-induced differentiation of human colon carcinoma cells. Oncogene Res 5:13–23, 1989
Marcu KB, Bossone SA, Patel AJ:Myc function and regulation. Annu Rev Biochem 61:809–860, 1992
Tsuboi K, Hirayoshi K, Takeuchi K, Sabe H, Shimada Y, Ohshio G, Tobe T, Hatanaka M: Expression of the c-myc gene in human gastrointestinal malignancies. Biochem Biophys Res Commun 146:669–704, 1987
Stewart J, Evan G, Watson J, Sikora K: Detection of the c-myc oncogene in colonic polyps and carcinomas. Br J Cancer 53:1–6, 1986
Finley GG, Schulz NT, Hill SA, Geiser JR, Pipas JM, Meisler AI: Expression of themyc gene family in different stages of human colorectal cancer. Oncogene 4:963–971, 1989
Untawale S, Blick M: Oncogene expression in adenocarcinomas of the colon and in colon tumour-derived cell lines. Anticancer Res 8:1–7, 1988
Dolcetti R, DeRe V, Viel A, Pistello M, Tavian M, Boicchi M, Meisler AI: Nuclear oncogene amplification or rearrangement is not involved in human colorectal malignancies. Eur J Cancer Clin Oncol 24:1321–1328, 1988
Taylor CW, Kim YS, Childress-Fields KE, Yeoman LC: Sensitivity of nuclear c-myc levels and induction to differentiation-inducing agents in human colon tumor cell lines. Cancer Lett 62:95–105, 1992
Barnard JA, Warwick G: Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ 4:495–501, 1993
Heruth DP, Zirnstein GW, Bradley JF, Rothberg PG: Sodium butyrate causes an increase in the block to transcriptional clongation in the c-myc gene in SW837 rectal carcinoma cells. J Biol Res 268:20466–20472, 1993
Souleimani A, Asselin C: Regulation of c-myc expression by sodium butyrate in the colon carcinoma cell line CaCo-2. FEBS Lett 326:45–50, 1993
Bohmann D, Bos TJ, Admon A, Nishimura T, Vogt PK, Tjian R: Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science 238:1386–1392, 1987
Souleimani A, Asselin C: Regulation of c-fos expression by sodium butyrate in the human colon carcinoma cell line CaCo-2. Biochem and Biophys Resch Commun 193(1):330–336, 1993
Toribara NW, Sack TL, Gum JR, Ho SB, Shively JE, Willson JKV, Kim YS: Heterogeneity in the induction and expression of carcinoembryonic antigen-related antigens in human colon cancer cell lines. Cancer Res 49:3321–3327, 1989
Heerdt BG, Augenlicht LH: Effects of fatty acid on expression of genes encoding subunits of cytochromec oxidase and cytochromec oxidase activity in HT29 human colonic adenocarcinoma cells. J Biol Chem 266:19120–19126, 1991
Hague A, Manning AM, Hanlon KA, Huschtscha LI, Hart D, Paraskeva C: Sodium butyrate induces apoptosis in the human colonic tumour cell line in a p-53 independent pathway: implications for the possible role of dietary fibre in the prevention of large bowel cancer. Int J Cancer 55:498–505, 1993
Kruh J, Tichonicky L, Defer N: Effect of butyrate on gene expression.In Short-Chain Fatty Acids. J Cummings, HJ Binder, K Soergel (eds). Boston, Kluwer Academic Publishers. 1994, pp 135–147
Boffa LC, Vidali G, Mann RS, Allfrey VG: Suppression of histone deacetylationin vivo andin vitro by sodium butyrate. J Biol Chem 253:3364–3366, 1978
Boffa L, Vidali G, Mann R, Allfrey V: Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem 256:9612–9621, 1981
Kruh J: Effect of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biol 42:65–82, 1982
Whitlock JP, Galcazzi D, Schulman H: Acetylation and calcium dependent phosphorylation of histone H3 in nuclei from butyrate-treated HeLa cells. J Biol Chem 258:1299–1304, 1983
Parker MI, de Haan JB, Gevers W: DNA hypermethylation in sodium byturate-treated WI-38 fibroblasts. J Biol Chem 261:2786–2790, 1985
Rastl E, Swetly P: Expression of poly-(adenosine diphosphate-ribose) polymerase activity in erythroleukemic mouse cells during cell cycle and erythropoietic differentiation. J Biol Chem 253:4333–4340, 1978
Cousenes LS, Gallwitz D, Alberts BM: Different accessibilities in chromatin to histone acetylase. J Biol Chem 254:1716–1723, 1979
Christman JK, Weich N, Schoenbrun B, Schneidman N, Acs G: Hypomethylation of DNA during differentiation of Friend erythroleukemia cells. J Cell Biol 86:366–370, 1980
Reeves R, Dserjesi P: Sodium butyrate induces new gene expression in Friend erythroleukemic cells. J Biol Chem 254:4383–4390, 1979
Vidali G, Boffa LC, Bradbury EM, Allfrey VG: Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histone H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci USA 75:2239, 1978
Deng G, Liu G, Hu L, Gum JR, Kim YS: Transcriptional regulation of the human placental-like alkaline phosphatase gene and mechanisms involved in its induction by sodium butyrate. Cancer Res 52:3378–3383, 1992
Willumsen BM, Christensen A, Hubbert NL, Papageorge AG, Lowy DR: The p21ras C-terminus is required for transformation and membrane association. Nature 310:583–586, 1984
Schafer WR, Kim R, Sterne R, Thorner J, Kim S, Rine J: Genetic and pharmacological suppression of oncogenic mutations inras genes of yeast and humans. Science 245:379–385, 1989
Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B: Prevalence ofras gene mutations in human colorectal cancers. Nature 327:293–297, 1987
Volgelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL: Genetic alterations during colorectal-tumor development. N Engl J Med 319:525, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Velázquez, O.C., Lederer, H.M. & Rombeau, J.L. Butyrate and the colonocyte. Digest Dis Sci 41, 727–739 (1996). https://doi.org/10.1007/BF02213129
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02213129