Abstract
Objective
To evaluate the effect of intravenous cefazolin on gastric emptying measured by the C-13 octanoic acid breath test.
Design
Prospective, double-blind, cross-over, randomised, placebo-controlled trial.
Setting
Mixed multidisciplinary intensive care unit in a university hospital.
Patients
Fourteen critically ill, mechanically ventilated patients.
Interventions
After a 4-h fast patients received either 50 mg cefazolin or 20 ml saline over 20 min immediately prior to measurement of gastric emptying. The next day the study was repeated with the alternative therapy.
Measurements and results
Breath samples were analysed for the concentration of 13CO2 by mass spectrometer, and the gastric emptying coefficient (GEC) and half-emptying time (t50) were calculated. Results are mean (standard deviation). Data were analysed with the paired t-test (saline vs cefazolin). Two patients were excluded for technical problems. Twelve patients remained (six male/six female), aged 57 (±16) years, with an APACHE II score of 20 (±8). Both GEC and t50 were unchanged after administration of cefazolin compared with placebo (t50 cefazolin, 138 (±54) vs saline 122 (±46) min, P=0.32; GEC cefazolin 3.27 (±0.83) vs saline 3.55 (±0.6), P=0.24). Two patients had abnormal t50 after saline and five after cefazolin. There was no order effect of the study day.
Conclusion
In mechanically ventilated patients, cefazolin had no effect on gastric emptying. These data do not support the use of low-dose cefazolin as a pro-kinetic agent in critically ill patients.
Similar content being viewed by others
Introduction
Maintaining adequate nutrition is important in critically ill patients; however, delivery of enteral nutrition is frequently unsuccessful [1], primarily because of delayed gastric emptying.
A number of pro-kinetic agents are available for the treatment of gastric stasis. Erythromycin accelerates gastric emptying and improves the success of feeding [2, 3]. However, the therapeutic window for erythromycin is narrow, higher doses can cause gastrointestinal side effects, and potential interactions with other medications limit its use. Effective pro-kinetics, with fewer side effects, would be advantageous in the management of delayed gastric emptying in the critically ill.
The cephalosporin antibiotics have recently been reported to have pro-kinetic activity in both humans and a mouse model [4, 5]. The cephalosporin antibiotic reported to have the greatest pro-kinetic effect and largest therapeutic window was cefazolin [5]. Cefazolin increased gastric emptying in the mouse model more than both erythromycin and metoclopramide. Whether this drug is effective in enterally fed patients in intensive care is unknown.
Measurement of gastric emptying in the intensive care unit is difficult. Breath tests have recently been developed and validated that allow the non-invasive measurement of gastric emptying [6]. This technique uses a naturally occurring non-radioactive isotope of carbon incorporated into octanoic acid, which is added to a test meal. When the meal is emptied from the stomach and digested in the intestine, the octanoic acid is metabolised to carbon dioxide and excreted in exhaled air [6]. Our group has previously reported on the incidence of delayed gastric emptying in intensive care unit (ICU) patients, using this technique [7]. Gastric emptying coefficient (GEC) was abnormal in 45% of patients when compared with volunteers.
The purpose of this study was to determine whether the antibiotic, cefazolin, accelerates gastric emptying in critically ill patients who are receiving enteral nutrition.
Materials and method
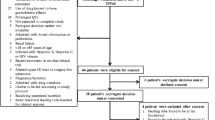
Fourteen mechanically ventilated patients aged 18 years or over were recruited from a mixed medical/surgical ICU. All patients were considered suitable for, or were receiving, enteral feeding, and had a nasogastric tube in situ.
Patients were excluded if there was:
-
Past history of gastrectomy or partial gastrectomy.
-
Use of cisapride within 48 h or metoclopramide or erythromycin within 24 h of the study.
-
Known allergy to cephalosporins or history of anaphylaxis associated with penicillin.
-
Use of any cephalosporin within 24 h of the study.
-
Pregnancy or breast feeding.
The Human Research Ethics Committee of the Royal Adelaide Hospital approved the protocol, which was conducted in accordance with NH&MRC guidelines for conduct of research in unconscious patients and with the 1964 declaration of Helsinki. Written informed consent was obtained from each patient's next of kin prior to enrolment.
The patients were studied on two consecutive days. On day 1, after a 4-h fast, patients received 100 ml of Ensure (liquid nutrition formulation) mixed with 100 μl octanoic acid labelled with 13C, which was instilled into the stomach via a nasogastric tube over 5 min. At the same time the patient was randomised to receive either intravenous cefazolin (50 mg) or intravenous saline, which was infused over 20 min. Breath samples were collected into vacutainers before the meal and every 5 min for the first hour and then every 15 min after the meal for 3 h and then stored for subsequent analysis.
The technique for gas sampling for 13C-labelled breath tests has been previously described in detail [7]. To permit the sampling of air from the ventilator tubing, a sidearm (StraightT adapter, Datex-Engstrom, Helsinki, Finland) was connected to the ventilation tubing. A holder for vacutainers (Blood needle holder, Reko, Lisarow, Australia) containing a needle (Venoject, Terumo Corporation, Tokyo, Japan) was attached. The vacutainers were pierced by the needle at the end of expiration. Percentage of CO2 in the sample, and fraction of 13C to total C, were measured by mass spectrometer. Samples were excluded if there was less than 1% CO2 present.
The following day, a second breath test was performed in the same manner, with the alternative infusion administered (double blind).
The GEC and half-emptying time (t50) were calculated in accordance with the method of Ghoos and Maes [6]. Normal ranges for these values have been previously determined in 22 volunteers [7] (GEC 3.2–3.8, t50 120–145 min; higher GEC and lower t50 indicates faster gastric emptying). Results are mean (± standard deviation). Data were analysed with the paired t-test (saline vs cefazolin).
Results
Fourteen patients were enrolled into the study. Unsatisfactory breath samples were collected in one subject, and one subject vomited half way through the second study, which left 12 for final analysis.
The study group comprised six men and six women with a mean age of 57 (±16) years and a mean admission APACHE II score of 20 (±8). Table 1 indicates the diagnostic categories of the patients and Table 2 shows associated risk factors for gastroparesis.
Cefazolin had no effect on gastric emptying when compared with saline (t50 cefazolin 138 (±54) min vs saline 122 (±46) min (Fig. 1), P=0.32; GEC cefazolin 3.27 (±0.83) vs saline 3.55 (±0.6), P=0.24). Although not statistically significant, there was a trend for the slowing of gastric emptying following administration of cefazolin. Two patients had abnormal t50 following administration of placebo and five following cefazolin. Of the two with delayed gastric emptying in the placebo group one improved after receiving cefazolin and one did not. There was no order effect of the study day on either t50 or GEC (P>0.5 for both).
There was no correlation between volumes of gastric aspirates, amount of feed delivered, amount of feed absorbed, and gastric emptying as measured by breath tests.
Discussion and conclusion
In contrast to previous reports, the results of this study suggest that cefazolin does not accelerate gastric emptying [4, 5]. However, not all studies that have examined the effect of cephalosporins on gastric emptying have shown a positive result. Cefaclor delayed gastric emptying by a cholecystokinin-mediated effect in a rat model [8]. Although cefazolin has been reported to increase gastric motility [4] it is possible for this to occur with a coincident slowing of gastric emptying due to increased tonicity of the pylorus [9].
Erythromycin in doses significantly lower than those required for an antibiotic effect has a substantial gastrointestinal effect [10]. Fifty milligrammes of cefazolin is well below the dose required for antibiotic effect; this dose has been reported to have a significant effect on human gastric motility [4]. However, it is possible that the failure of cefazolin to increase gastric emptying in the current study is because the dose was sub-therapeutic.
The incidence of delayed gastric emptying in this study was 17%, considerably less than the 45% reported in a previous study of unselected critically ill patients [7]. This probably reflects the exclusion of patients who had required pro-kinetics prior to the study. In line with the aggressive feeding policy of the unit, patients receive pro-kinetics with the first evidence of failure to tolerate feeding. Thus, patients likely to have delayed gastric emptying were unintentionally excluded from the trial. In a previous study that examined the effect of erythromycin on gastric emptying, an improvement was significant only in patients who had slow baseline gastric emptying [10].
Although it is possible that the breath test lacks sufficient sensitivity to detect changes in gastric emptying induced by cefazolin, this appears unlikely. Previous studies by the authors have shown that the breath test can be used effectively in this group of patients [7], that gastric emptying measured by this technique is delayed in the critically ill [7], and that erythromycin improves gastric emptying in an unselected group of critically ill patients [10].
The power of this study to detect a difference between the t50 of the two groups of 30 min was only 46%; however, the trend towards slowed gastric emptying following the administration of cefazolin suggests that further studies with either increased numbers or the use of a more sensitive test are not warranted.
In conclusion, we found that in mechanically ventilated critically ill patients, low-dose cefazolin had no effect on gastric emptying. These data do not support the use of low-dose cefazolin as a pro-kinetic agent in critically ill patients.
References:
de Beaux I, Chapman M, Fraser R, Finnis M, De Keulenaer B, Liberalli D, Satanek M (2001) Enteral nutrition in the critically ill: a prospective survey in an Australian intensive care unit. Anaesth Intensive Care 29:619–622
Chapman MJ, Fraser RJ, Kluger MT, Buist MD, De Nichilo DJ (2000) Erythromycin improves gastric emptying in critically ill patients intolerant of nasogastric feeding. Crit Care Med 28:2334–2337
Fraser R, Shearer T, Fuller J, Horowitz M, Dent J (1992) Intravenous erythromycin overcomes small intestinal feedback on antral, pyloric and duodenal motility. Gastroenterology 103:114–119
Lamport RD, Thompson WG, Schuster MM (1995) The effects of erythromycin and Ancef on gastric and small bowel motility in patients with gastrointestinal dysmotility. Am J Gastroenterol 90:1633
Kuo WH, Wadwa KS, Ferris CD (1998) Cephalosporin antibiotics accelerate gastric emptying in mice. Dig Dis Sci 43:1690–1694
Maes BD, Ghoos YF, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G (1994) Combined carbon-13-glycine/carbon-14-octanoic acid breath test to monitor gastric emptying rates of liquids and solids. J Nucl Med 35:824–831
Ritz MA, Fraser R, Edwards N, Di Matteo AC, Chapman M, Butler R, Cmielewski P, Tournadre JP, Davidson G, Dent J (2001) Delayed gastric emptying in ventilated critically ill patients: measurement by 13C-octanoic acid breath test. Crit Care Med 29:1744–1749
Bozkurt A, Deniz M, Yegen BC (2000). Cefaclor, a cephalosporin antibiotic, delays gastric emptying rate by a CCK-A receptor-mediated mechanism in the rat. Br J Pharmacol 131:399–404
Fraser R, Fone D, Horowitz M, Dent J (1993). Cholecystokinin octapeptide stimulates phasic and tonic pyloric motility in healthy humans. Gut 34:33–37
Ritz M, Fraser R, Chapman M, Butler R, Cmielewski P, Davidson G, Rea DA, Dent J (1999). Two different doses of erythromycin in the treatment of delayed gastric emptying in critically ill patients. Neurogastroenterol Motil 11:205
Acknowledgments
This work was supported by a project grant from the National Health and Medical Research Council, Australia, and the Special Purposes Fund of the Royal Adelaide Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chapman, M., Fraser, R., de Beaux, I. et al. Cefazolin does not accelerate gastric emptying in the critically ill. Intensive Care Med 29, 1169–1172 (2003). https://doi.org/10.1007/s00134-003-1803-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1803-2