Abstract
Hydrogen sulfide (H2S) is present in the lumen of the human large intestine at millimolar concentrations. However, the concentration of free (unbound) sulfide is in the micromolar range due to a large capacity of fecal components to bind the sulfide. H2S can be produced by the intestinal microbiota from alimentary and endogenous sulfur-containing compounds including amino acids. At excessive concentration, H2S is known to severely inhibit cytochrome c oxidase, the terminal oxidase of the mitochondrial electron transport chain, and thus mitochondrial oxygen (O2) consumption. However, the concept that sulfide is simply a metabolic troublemaker toward colonic epithelial cells has been challenged by the discovery that micromolar concentration of H2S is able to increase the cell respiration and to energize mitochondria allowing these cells to detoxify and to recover energy from luminal sulfide. The main product of H2S metabolism by the colonic mucosa is thiosulfate. The enzymatic activities involved in sulfide oxidation by the colonic epithelial cells appear to be sulfide quinone oxidoreductase considered as the first and rate-limiting step followed presumably by the action of sulfur dioxygenase and rhodanese. From clinical studies with human volunteers and experimental works with rodents, it appears that H2S can exert mostly pro- but also anti-inflammatory effects on the colonic mucosa. From the available data, it is tempting to propose that imbalance between the luminal concentration of free sulfide and the capacity of colonic epithelial cells to metabolize this compound will result in an impairment of the colonic epithelial cell O2 consumption with consequences on the process of mucosal inflammation. In addition, endogenously produced sulfide is emerging as a prosecretory neuromodulator and as a relaxant agent toward the intestinal contractibility. Lastly, sulfide has been recently described as an agent involved in nociception in the large intestine although, depending on the experimental design, both pro- and anti-nociceptive effects have been reported.



Similar content being viewed by others
References
Ahmad T, Satsangi J, McGovern D, Bunce M, Jewell DP (2001) Review article: the genetics of inflammatory bowel disease. Aliment Pharmacol Ther 15:731–748
Allen A, Flemström G (2005) Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol 288:C1–C19
Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR (2006) Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res 4:9–14
Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ (2007) Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res 5:455–459
Awano N, Wada M, Mori H, Nakamori S, Takagi H (2005) Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl Environ Microbiol 71:4149–4152
Babidge W, Millard S, Roediger W (1998) Sulfides impair short chain fatty acid beta-oxidation at acyl-CoA dehydrogenase level in colonocytes: implications for ulcerative colitis. Mol Cell Biochem 181:117–124
Baglieri A, Mahe S, Zidi S, Huneau JF, Thuiller F, Marteau P, Tome D (1994) Gastro-jejunal digestion of soya-bean-milk protein in humans. Br J Nutr 72:519–532
Blachier F, Mariotti F, Huneau JF, Tome D (2007) Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 33:547–562
Bos C, Juillet B, Fouillet H, Turlan L, Dare S, Luengo C, Ntounda R, Benamouzig R, Gausseres N, Tome D, Gaudichon C (2005) Postprandial metabolic utilization of wheat protein in humans. Am J Clin Nutr 81:87–94
Buttgereit F, Brand MD (1995) A hierarchy of ATP-consuming processes in mammalian cells. Biochem J 312:163–167
Chacko A, Cummings JH (1988) Nitrogen losses from the human small bowel: obligatory losses and the effects of physical form of food. Gut 29:809–815
Christl SU, Gibson GR, Cummings JH (1992) Role of dietary sulphate in the regulation of methanogenesis in the human large intestine. Gut 33:1234–1238
Christl SU, Eisner HD, Dusel G, Kasper H, Scheppach W (1996) Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa: a potential role for these agents in the pathogenesis of ulcerative colitis. Dig Dis Sci 41:2477–2481
Clauss MT, Zocher GE, Maier THP, Schulz GE (2005) Structure of the O-acetyl-serine sulfurhydralase, 2 isoenzyme CYSM from Escherichia coli. Biochem 44:8620–8626
Deplancke B, Gaskins HR (2003) Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J 17:1310–1312
Deplancke B, Finster K, Graham WV, Collier CT, Thurmond JE, Gaskins HR (2003) Gastrointestinal and microbial responses to sulfate-supplemented drinking water in mice. Exp Biol Med 228:424–433
Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, Fiorucci S (2006a) Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther 316:325–335
Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, Caliendo G, Santagada V, Cirino G, Wallace JL, Fiorucci S (2006b) 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-(1, 2)dithiol-3-yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther 319:447–458
Drassar BS, Hill MJ (1974) Human Intestinal Flora. Academic Press, London
Edmond LM, Hopkins MJ, Magee EA, Cummings JH (2003) The effect of 5-aminosalicylic acid-containing drugs on sulfide production by sulfate-reducing and amino acid-fermenting bacteria. Inflamm Bowel Dis 9:10–17
Evenepoel P, Claus D, Geypens B, Hiele M, Geboes K, Rutgeerts P, Ghoos Y (1999) Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am J Physiol 277:G935–G943
Florin T (1991) Hydrogen sulphide and total acid-volatile sulphide in faeces, determined with a direct spectrophotometric method. Clin Chim Acta 196:127–134
Florin T, Neale G, Gibson GR, Christl SU, Cummings JH (1991) Metabolism of dietary sulphate: absorption and excretion in humans. Gut 32:766–773
Furne JK, Suarez FL, Ewing SL, Springfield J, Levitt MD (2000) Binding of hydrogen sulfide by bismuth does not prevent dextran sulfate-induced colitis in rats. Dig Dis Sci 45:1439–1443
Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD (2001) Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol 62:255–259
Gallego D, Clavé P, Donovan J, Rahmati R, Grundy D, Jimenez M, Beyak MJ (2008) The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil 20:1306–1316
Gaudichon C, Mahe S, Benamouzig R, Luengo C, Fouillet H, Dare S, Van Oycke M, Ferriere F, Rautureau J, Tome D (1999) Net postprandial utilization of (15N)-labeled milk protein nitrogen is influenced by diet composition in humans. J Nutr 129:890–895
Gaudichon C, Bos C, Morens C, Petzke KJ, Mariotti F, Everwand J, Benamouzig R, Dare S, Tome D, Metges CC (2002) Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterology 123:50–59
Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R (1999) Dextran sulfate sodium colitis in rats: clinical structural, and ultrastructural aspects. Dig Dis Sci 44:1458–1475
Gausseres N, Mahe S, Benamouzig R, Luengo C, Drouet H, Rautureau J, Tome D (1996) The gastro-ileal digestion of 15-N labelled pea nitrogen in adult humans. Br J Nutr 76:75–85
Gibson JA, Sladem GE, Dawson AM (1976) Protein absorption and ammonia production: the effects of dietary protein and removal of the colon. Br J Nutr 35:61–65
Gibson GR, Cummings JH, Macfarlane GT (1988a) Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine. J Appl Bacteriol 65:241–247
Gibson GR, Cummings JH, Macfarlane GT (1988b) Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol 54:2750–2755
Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F (2007) Sulfide, the first inorganic substrate for human cells. FASEB J 21:1699–1706
Griesbeck C, Schütz M, Schödl T, Bathe S, Nausch L, Mederer N, Vielreicher M, Hauska G (2002) Mechanism of sulfide-quinone reductase investigated using site-directed mutagenesis and sulfur analysis. Biochemistry 41:11552–11565
Grieshaber MK, Völkel S (1998) Animal adaptations for tolerance and exploitation of poisonous sulfide. Annu Rev Physiol 60:33–53
Hedderich R, Klimmek O, Kroëger A, Dirmeier R, Keller M, Stetter KO (1999) Anaerobic respiration with elemental sulfur and with disulfides. FEMS Microbiol Rev 22:353–381
Hildebrand TM, Grieshaber MK (2008) Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and inverterbrate mitochondria. FEBS J 275:3352–3361
Hill BC, Woon TC, Nicholls P, Peterson J, Greenwood C, Thomson AJ (1984) Interactions of sulphide and other ligands with cytochrome c oxidase. Biochem J 224:591–600
Jorgensen J, Mortensen PB (2001) Hydrogen sulfide and colonic epithelial metabolism: implications for ulcerative colitis. Dig Dis Sci 46:1722–1732
Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, Welfare MR (2004) Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut 53:1479–1484
Kadota H, Ishida Y (1972) Production of volatile sulfur compounds by microorganisms. Ann Rev Microbiol 26:127–138
Kallio RE (1951) Function of pyridoxal phosphate in desulfhydrase systems of Proteus morganii. J Biol Chem 192:371–377
Kanazawa K, Konishi F, Mitsuoka T, Terada A, Itoh K, Narushima S, Kumemura M, Kimura H (1996) Factors influencing the development of sigmoid colon cancer. Bacteriologic and biochemical studies. Cancer 77:1701–1706
Kramer P (1966) The effect of varying sodium loads on the ileal excreta of human ileostomised subjects. J Clin Invest 45:1710–1718
Kumagai H, Sejima S, Choi Y, Tanaka H, Yamada H (1975) Crystallization and properties of cysteine desulfhydrase from Aerobacter aerogenes. FEBS Lett 52:304–307
Leschelle X, Goubern M, Andriamihaja M, Blottière HM, Couplan E, Gonzales-Barroso M, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F (2005) Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta 1725:201–212
Leung FW, Heng MC, Allen S, Seno K, Leung JWC, Heng MK (2000) Involvement of luminal bacteria, heat shock protein 60, macrophages and gammadelta T cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci 45:1472–1479
Levine J, Ellis CJ, Furne JK, Spingfield J, Levitt MD (1998) Fecal hydrogen sulfide production in ulcerative colitis. Am J Gastroenterol 93:83–87
Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E (1999) Detoxication of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest 104:1107–1114
Levitt MD, Springfield J, Furne J, Koenig T, Suarez FL (2002) Physiology of sulfide in the rat colon: use of bismuth to assess colonic sulfide production. J Appl Physiol 92:1655–1660
Lewis S, Cochrane S (2007) Alteration of sulfate and hydrogen metabolism in the human colon by changing intestinal transit rate. Am J Gastroenterol 102:624–633
Liau YH, Horowitz MI (1976) The importance of PAPS in determining sulphation in gastrointestinal mucosa. Digestion 14:372–375
Linden DR, Sha L, Mazzone A, Stolz GJ, Bernard CE, Furne JK, Levitt MD, Farrugia G, Szurszewski JH (2008) Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem 106:1577–1585
Loubinoux J, Bronowicki JP, Pereira IA, Mougenel JL, Faou AE (2002) Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol 40:107–112
Macfarlane S, Macfarlane GT (2003a) Food and the large intestine. In: Fuller R, Perdigon G (eds) Gut flora, nutrition, immunity and health. Blackwell, Oxford, pp 24–51
Macfarlane S, Macfarlane GT (2003b) Regulation of short-chain fatty acid production. Proc Nutr Soc 62:67–72
Macfarlane GT, Gibson GR, Cummings JH (1992) Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72:57–64
Magee EA, Richardson CJ, Hughes R, Cummings JH (2000) Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr 72:1488–1494
Matsunami M, Tarui T, Mitani K, Nagasawa K, Fukushima O, Okubo K, Yoshida S, Takemura M, Kawabata A (2009) Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut 58:751–761
Mitsui T, Edmond LM, Magee EA, Cummings JH (2003) The effects of bismuth, iron, zinc and nitrate on free sulfide in batch cultures seeded with fecal flora. Clin Chim Acta 335:131–135
Moore JW, Babidge W, Millard S, Roediger WEW (1997) Effect of sulphide on short chain acyl-CoA metabolism in rat colonocytes. Gut 41:77–81
Moore J, Babidge W, Millard S, Roediger W (1998) Colonic luminal hydrogen sulfide is not elevated in ulcerative colitis. Dig Dis Sci 43:162–165
Nicholson RA, Roth SH, Zhang A, Zheng J, Brookes J, Skrajny B, Bennington R (1998) Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. J Toxicol Environ Health 54:491–507
Petersen LC (1977) The effects of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta 460:299–307
Picton R, Eggo MC, Merrill GA, Langman MJS, Singh S (2002) Mucosal protection against sulphide: importance of the enzyme rhodanese. Gut 50:201–205
Picton R, Eggo MC, Langman MJ, Singh S (2007) Impaired detoxication of hydrogen sulfide in ulcerative colitis? Dig Dis Sci 52:373–378
Pitcher MC, Pitcher JM (1996) Hydrogen sulfide: a bacterial toxin in ulcerative colitis? Gut 39:1–4
Pitcher MC, Beatty ER, Cummings JH (2000) The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut 46:64–72
Pochart P, Doré J, Lémann F, Goderel I, Rambaud JC (1992) Interrelations between populations of methanogenic archae and sulfate-reducing bacteria in the human colon. FEMS Microbiol Lett 77:225–228
Rabus R, Hansen TH, Widdel F (2006) Dissimilatory sulfate- and sulfur-reducing prokaryotes. In: Dworkin M (ed) The prokaryotes. A handbook of the biology of bacteria: symbiotic associations, Biotechnology, Applied Microbiology, 3rd edn. Springer, New York, 2:659–768
Ramasamy S, Singh S, Taniere P, Langman MJS, Eggo MC (2006) Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am J Physiol 291:G288–G296
Reiffenstein RJ, Hulbert WC, Roth SH (1992) Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32:109–134
Roediger WEW, Duncan A, Kapanaris O, Millard S (1993a) Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology 104:802–809
Roediger WEW, Duncan A, Kapanaris O, Millard S (1993b) Sulphide impairment of substrate oxidation in rat colonocytes: a biochemical basis for ulcerative colitis? Clin Sci 85:623–627
Roediger WE, Babidge W, Millard S (1996) Methionine derivatives diminish sulphide damage to colonocytes: implications for ulcerative colitis. Gut 39:77–81
Scanlan PD, Shanahan F, Marchesi JR (2009) Culture-independent analysis of desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. FEMS Microbiol Ecol 69:213–221
Schauder R, Kröger A (1993) Bacterial sulphur respiration. Arch Microbiol 159:491–497
Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M (2006) Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology 131:1542–1552
Shaw L, Engel PC (1987) CoA-persulphide: a possible in vivo inhibitor of mammalian short-chain acyl-CoA dehydrogenase. Biochim Biophys Acta 919:171–174
Siegel LM, Murphy MJ, Kamin H (1973) Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of Enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem 248:251–264
Silvester KR, Cummings JH (1995) Does digestibility of meat protein help explain large bowel cancer risk? Nutr Cancer 24:279–288
Smiddy FG, Gregory SD, Smith IB, Goligher JC (1960) Fecal loss of fluid, electrolytes and nitrogen in colitis before and after ileostomy. Lancet 1:14–19
Smith EA, Marcfarlane GT (1997) Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 3:327–337
Stewart JA, Chadwick VS, Murray A (2006) Carriage, quantification, and predominance of methanogens and sulfate-reducing bacteria in faecal samples. Lett Appl Microbiol 43:58–63
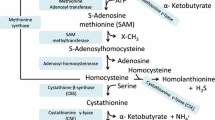
Stipanuk MH (2004) Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24:539–577
Strocchi A, Furne J, Ellis C, Levitt MD (1994) Methanogens outcompete sulphate-reducing bacteria for H2 in the human colon. Gut 35:1098–1101
Suarez F, Furne J, Springfield J, Levitt M (1998) Production and elimination of sulfur-containing gases in the rat colon. Am J Physiol 274:G727–G733
Szabo C (2007) Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6:917–935
Tai CH, Burkhard P, Gani D, Jenn T, Johnson C, Cook PF (2001) Characterization of the allosteric anion-binding site of O-acetylserine sulfhydrylase. Biochem 40:7446–7452
Tamaru T, Kobayashi H, Kishimoto S, Kajiyama G, Shimamoto F, Brown WR (1993) Histochemical study of colonic cancer in experimental colitis in rats. Dig Dis Sci 38:529–537
Taniguchi E, Matsunami M, Kimura T, Yonezawa D, Ishiki T, Sekiguchi F, Nishikawa H, Maeda Y, Ishikura H, Kawabata A (2009) Rhodanese but not cystathionine-gamma-lyase is associated with dextran sulfate sodium-evoked colitis in mice: a sign of impaired colonic sulfide detoxification? Toxicology 264:96–103
Teague B, Asiedu S, Moore PK (2002) The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractibility. Br J Pharmacol 137:139–145
Tiranti V, Viscomi C, Hildebrand T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, Zeviani M (2009) Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med 15:200–205
Wallace JL, Vong L, McKnight W, Dicay M, Martin GR (2009) Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137:569–578
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798
Watt J, Marcus R (1973) Experimental ulcerative diseases of the colon in animals. Gut 14:506–510
Wedzicha BL (1984) Chemistry of sulphur dioxide in foods. Elsevier Applied Science Publishers, London
Weisiger RA, Pinkus LM, Jakoby WB (1980) Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol 29:2885–2887
Willis CL, Cummings JH, Neale G, Gibson GR (1996) In vitro effects of mucin fermentation on the growth of human colonic sulphate-reducing bacteria. Anaerobe 2:117–122
Willis CL, Cummings JH, Neale G, Gibson GR (1997) Nutritional aspects of dissimilatory sulfate reduction in the human large intestine. Curr microbiol 35:294–298
Wilson TH (1962) Intestinal absorption. WB Saunders, Philadelphia, pp 134–137
Wilson K, Mudra M, Furne J, Levitt M (2008) Differentiation of the roles of sulfide oxidase and rhodanese in the detoxication of sulfide by the colonic mucosa. Dig Dis Sci 53:277–283
Xu GY, Winston JH, Shenoy M, Zhou S, Chen JD, Pasricha PJ (2009) The endogenous hydrogen sulfide producing enzyme cystathionine-beta synthase contributes to visceral hypersensitivity in a rat model of irritable bowel syndrome. Mol Pain 5:44
Yoshikawa S (1999) X-ray structure and reaction mechanism of bovine heart cytochrome c oxidase. Biochem Soc Trans 27:351–362
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blachier, F., Davila, AM., Mimoun, S. et al. Luminal sulfide and large intestine mucosa: friend or foe?. Amino Acids 39, 335–347 (2010). https://doi.org/10.1007/s00726-009-0445-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0445-2