Abstract
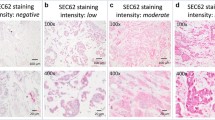
CUB and SUSHI multiple domain protein 1 (CSMD1) is a candidate tumour suppressor gene that maps to chromosome 8p23, a region deleted in many tumour types including 50% of breast cancers. CSMD1 has homologies to proteins implicated in carcinogenesis. We aimed to study the expression pattern of the CSMD1 protein and evaluate its prognostic importance in invasive ductal carcinoma (IDC). An anti-CSMD1 antibody was developed and validated. The expression pattern of CSMD1 in normal breast and IDC samples was investigated by immunohistochemistry in 275 patients. Univariate and multivariate Cox regression analyses were performed. In normal breast duct epithelial cells, luminal, membranous and cytoplasmic CSMD1 staining was identified. Reduced expression of CSMD1 was detected in 79/275 (28.7%) of IDC cases. Low CSMD1 expression was significantly associated with high tumour grade (P = 0.003). CSMD1 expression was associated with overall survival (OS; HR = 0.607, 95%CI: 0.4–0.91, P = 0.018) but not with disease-free survival (DFS; HR = 0.81, 95%CI: 0.46–1.43, P = 0.48). Multivariate analysis showed that CSMD1, together with Nottingham Prognostic Index, was considered an independent predictor of OS (HR = 0.607, 95%CI: 0.4–0.91, P = 0.018) but not DFS (HR = 0.84, 95%CI: 0.46–1.5, P = 0.573). Reduction of CSMD1 expression was significantly associated with high tumour grade and decreased OS. Therefore, our results support the idea that CSMD1 is a tumour suppressor gene and suggest its possible use as a new prognostic biomarker. The membrane expression pattern of CSMD1 suggests that it may be a receptor or co-receptor involved in the process of signal transduction.



Similar content being viewed by others
References
Bray F, Sankila R, Ferlay J et al (2002) Estimates of cancer incidence, mortality in Europe in 1995. Eur J Cancer 38(1):99–166
Parkin DM, Bray F, Ferlay J et al (2001) Estimating the world cancer burden: globocan 2000. Int J Cancer 94(2):153–156
Fabre-Lafay S, Monville F, Garrido-Urbani S et al (2007) Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 7(1):73
Alford D, Taylor-papadimitrion J (1996) Cell adhesion molecules in the normal and cancerous mammary gland. J Mammary Gland Biol Neoplasia 1(2):207–218
Sun PC, Uppaluri R, Schmidt AP et al (2001) Transcript map of the 8p23 putative tumor suppressor region. Genomics 75(1–3):17–25
Kirkitadze MarinaD, NB P (2001) Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol Rev 180(1):146–161
Sigbjornsdottir BI, Ragnarsson G, Agnarsson BA et al (2000) Chromosome 8p alterations in sporadic and BRCA2 999del5 linked breast cancer. J Med Genet 37(5):342–347
Yokota T, Yashimoto M, Akiyama F et al (1999) Localization of a tumor suppressor gene associated with the progression of human breast carcinoma within a 1-cm interval of 8p22–p23.1. Cancer 85(2):447–452
Emi M, Fujiwara Y, Nakajima T et al (1992) Frequent loss of heterozygosity for loci on chromosome 8p in hepatocellular carcinoma, colorectal cancer, and lung cancer. Cancer Res 52(19):5368–5372
Wu CL, Roz L, Sloan P et al (1997) Deletion mapping defines three discrete areas of allelic imbalance on chromosome arm 8p in oral and oropharyngeal squamous cell carcinomas. Genes. Chromosom Cancer 20(4):347–353
Toomes C, Jackson A, Maguire K et al (2003) The presence of multiple regions of homozygous deletion at the CSMD1 locus in oral squamous cell carcinoma question the role of CSMD1 in head and neck carcinogenesis. Genes. Chromosom Cancer 37(2):132–140
Sunwoo JB, Holt MS, Radford DM et al (1996) Evidence for multiple tumor suppressor genes on chromosome arm 8p in supraglottic laryngeal cancer. Genes Chromosomes Cancer 16(3):164–169
Scholnick SB, Haughey BH, Sunwoo JB et al (1996) Chromosome 8 allelic loss and the outcome of patients with squamous cell carcinoma of the supraglottic larynx. J Natl Cancer Inst 88(22):1676–1682
Fujiwara Y, Emi M, Ohata H et al (1993) Evidence for the presence of two tumor Suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res 53(5):1172–1174
Ishwad CS, Shuster M, Buckmuhl U et al (1999) Frequent allelic loss and homozygous deletion in chromosome band 8p23 in oral cancer. Int J Cancer 80(1):25–31
Ma C, Quesnelle KM, Sparano A et al (2009) Characterization CSMD1 in a large set of primary lung, head and neck, breast and skin cancer tissues. Cancer Biol Ther 8(10):29–38
Midorikawa Y, Yamamato S, Tsuji S et al (2008) Allelic imbalances and homozygous deletion on 8p23.2 for stepwise progression of hepatocarcinogenesis. Hepatology 49(2):513–522
Farrell LC, Crimm H, Meeh P et al (2008) Somatic mutations to CSMD1 in colorectal adenocarcinomas. Cancer Biol Ther 7(4):609–613
Henshall SM, Afar DEH, Hiller J et al (2003) Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res 63(14):4196–4203
Paris PL, Andaya A, Fridlyand J et al (2004) Whole genome scanning identifies genotypes associated with recurrence and metastasis in prostate tumors. Hum Mol Genet 13(13):1303–1313
Nishioka M, Kohno T, Takahashi M et al (2000) Identification of a 428-kb homozygously deleted region disrupting the SEZ6L gene at 22q12.1 in a lung cancer cell line. Oncogene 19(54):6251–6260
Kraus DM, Elliott GS, Chute H et al (2006) CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J Immunol 176(7):4419–4430
Mollenhauer J, Wiemann S, Scheurlen W et al (1997) DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3–26.1 is deleted in malignant brain tumours. Nat Genet 17(1):32–39
Wu W, Kemp BL, Proctor ML et al (1999) Expression of DMBT1, a candidate tumor suppressor gene, is frequently lost in lung cancer. Cancer Res 59(8):1846–1851
Mollenhauer J, Helmke B, Medina D et al (2004) Carcinogen inducibility in vivo and down-regulation of DMBT1 during breast carcinogenesis. Genes Chromosom Cancer 39(3):185–194
Bork P, Beckmann G (1993) The CUB domain: a widespread module in developmentally regulated proteins. J Mol Biol 231(2):539–545
Topfer-Petersen E, Romero A, Varela PF et al (1998) Spermadhesins: a new protein family. Facts, hypotheses and perspectives. Andrologia 30(4–5):217–224
Lu S-L, Kawabata M, Imamura T et al (1998) HNPCC associated with germline mutation in the TGF-[beta] type II receptor gene. Nat Genet 19(1):17–18
Su GH, Bansal R, Murphy KM et al (2001) ACVR1B (ALK4, activin receptor type 1B) gene mutations in pancreatic carcinoma. Proc Natl Acad Sci U S A 98(6):3254–3257
Williams LM, Drew JE, Bunnett NW et al (2001) Characterization of an antibody to the human melatonin mt1 receptor. J Neuroendocrinol 13(1):94–101
Muscheck M, Sukosd F, Pesti T et al (2000) High density deletion mapping of bladder cancer localizes the putative tumor suppressor gene between loci D8S504 and D8S264 at chromosome 8p23.3. Lab Invest 80(7):1089–1093
Washburn JG, Wojno KJ, Dey J et al (2000) 8pter-p23 deletion is associated with racial differences in prostate cancer outcome. Clin Cancer Res 6(12):4647–4652
Bockmühl U, Ishwad CS, Ferrell RE et al (2001) Association of 8p23 deletions with poor survival in head and neck cancer. Otolaryngol Head Neck Surg 124(4):451–455
Weber-Mangal S, Sinn H, Popp S et al (2003) Breast cancer in young women (< or =35 years): genomic aberrations detected by comparative genomic hybridization. Int J Cancer 107(4):583–592
Wright K, Wilson PJ, Kerr J et al (1998) Frequent loss of heterozygosity and three critical regions on the short arm of chromosome 8 in ovarian adenocarcinomas. Oncogene 17(9):1185–1188
Paccaud JP, Reith W, Johansson B et al (1993) Clathrin-coated pit-mediated receptor internalization. Role of internalization signals and receptor mobility. J Biol Chem 268(31):23191–23196
Richter T, Tong B, Scholnick S (2005) Epigenetic inactivation and aberrant transcription of CSMD1 in squamous cell carcinoma cell lines. Cancer Cell Int 5(1):29
Acknowledgments
This study was supported by grants from Yorkshire Cancer Research and Breast Cancer Campaign (SMB & VS), the Association for International Cancer Research (LZ & NT) and the Egyptian government (MK). CT is a Royal Society University Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamal, M., Shaaban, A.M., Zhang, L. et al. Loss of CSMD1 expression is associated with high tumour grade and poor survival in invasive ductal breast carcinoma. Breast Cancer Res Treat 121, 555–563 (2010). https://doi.org/10.1007/s10549-009-0500-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0500-4