Abstract
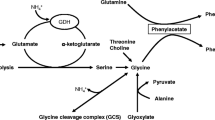
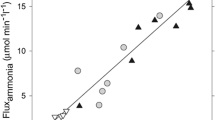
Systemic hyperammonemia has been largely found in patients with cirrhosis and hepatic encephalopathy, and ammonia plays a major role in the pathogenesis of hepatic encephalopathy. However, controversial points remain: a) the correlation between plasma ammonia levels and neurophysiological impairment. The lack of correlation between ammonia levels and grade of hepatic encephalopathy in some cases has been considered a weakness of the ammonia hypothesis, but new methods for ammonia measurements and the implication of systemic inflammation in the modulation of ammonia neurotoxicity could explain this gap; b) the source of ammonia production. Hyperammonemia has been considered as derived from urea breakdown by intestinal bacteria and the majority of treatments were targeted against bacteria-derived ammonia from the colon. However, some data suggest an important role for small intestine ammonia production: 1) the hyperammonemia after porto-caval shunted rats has been found similar in germ-free than in non-germ-free animals. 2) In cirrhotic patients the greatest hyperammonemia was found in portal drained viscera and derived mainly from glutamine deamination. 3) The amount of time required to increase of ammonia (less than one hour) after oral glutamine challenge supports a small intestine origin of the hyperammonemia. As the main source of ammonia in cirrhotics derives from portal drained viscera owing to glutamine deamidation, increased glutaminase activity in the intestine seems to be responsible for systemic hyperammonemia. Lastly, some genetic alterations in the glutaminase gene such as the haplotype TACC could modulate intestinal ammonia production and the risk of overt hepatic encephalopathy in cirrhotics.




Similar content being viewed by others
References
Albrecht J, Norenberg MD (2006) Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44:788–794
Bernal W, Donaldson P, Underhill J, Wendon J, Williams R (1998) Tumor necrosis factor genomic polymorphism and outcome of acetaminophen (paracetamol)-induced acute liver failure. J Hepatol 29:53–59
Campos J, Gonzalez-Quintela A, Quinteiro C, Gude F, Perez LF, Torre JA, Vidal C (2005) The −159C/T polymorphism in the promoter region of the CD14 gene is associated with advanced liver disease and higher serum levels of acute-phase proteins in heavy drinkers. Alcohol Clin Exp Res 29:1206–1213
Cauli O, Rodrigo R, Piedrafita B, Boix J, Felipo V (2007) Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology 46:514–519
Corvera S, Garcia-Sainz JA (1983) Hormonal stimulation of mitochondrial glutaminase. Effects of vasopressin, angiotensin II, adrenaline and glucagons. Biochem J 210:957–960
Curthoys NP, Watford M (1995) Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 15:133–59
Elgadi KM, Meguid RA, Qian M, Souba WW, Abcouwer SF (1999) Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol Genomics 1:51–62
Gabuzda GJ Jr, Phillips GB, Davidson CS (1952) Reversible toxic manifestations in patients with cirrhosis of the liver given cation-exchange resins. N Engl J Med 246:124–130
Hashimoto N, Ashida H, Kotoura Y, Nishioka A, Nishiwaki M, Utsunomiya J (1993) Analysis of hepatic encephalopathy after distal splenorenal shunt—PTP image and pancreatic hormone kinetics. Hepatogastroenterology 40:360–364
Häussinger D, Laubenberger J, vom Dahl S, Ernst T, Bayer S, Langer M, Gerok W, Hennig J (1994) Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology 107:1475–1480
Hawkins RA, Jessy J, Mans AM, Chedid A, DeJoseph MR (1994) Neomycin reduces the intestinal production of ammonia from glutamine. Adv Exp Med Biol 368:125–134
Jalan R, Kapoor D (2004) Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond) 106:467–474
James LA, Lunn PG, Elia M (1988) Glutamine metabolism in the gastrointestinal tract of the rat assessed by the relative activity of gutaminase (EC 3.5.1.2) and glutamine synthetase (EC 6.3.1.2). Br J Nutrition 79:365-372
James LA, Lunn PG, Middleton S, Elia M (1998) Distribution of glutaminase and glutamine synthase activities in the human gastrointestinal tract. Clin Sci 94:313-319
Jover M, Díaz D, Collantes-de-Terán L, Córpas R, Fontiveros E, Parrado J, Bautista JD, Romero-Gómez M (2005) Glutaminase activity is implicated in the pathogenesis of hyperammonemia in porto-caval shunted rats. J Hepatol 42(suppl 1):168A
Lewontin RC, Kojima K (1960) The evolutionary dynamics of complex olymorphisms. Evolution 14:450–462
Lockwood AH (2004) Blood ammonia levels and hepatic encephalopathy. Metab Brain Dis 19:345–349
Lockwood AH (2007) Controversies in ammonia metabolism: implications for hepatic encephalopathy. Metab Brain Dis 22:285–289
Malaguarnera M, Greco F, Barone G, Gargante MP, Malaguarnera M, Toscano MA (2007) Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci 52:3259–3265
Modi WS, Pollock DD, Mock BA, Banner C, Renauld JC, Van Snick J (1991) Regional localization of the human glutaminase (GLS) and interleukin-9 (IL9)genes by in situ hybridization. Cytogenet Cell Genet 57:114–116
Nance FC, Kline DG (1971) Eck’s fistula encephalopathy in germ-free dogs. Ann Surg 174:856–861
Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB (2002) Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology 36:1163–1171
Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, Arroliga AC, Mullen KD (2003) Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 114(3):188–193 Feb 15
Romero Gomez M, Bautista JD, Grande L, Ramos Guerrero RM, Sanchez Munoz D (2004) New concepts in the physiopathology of hepatic encephalopathy and therapeutic prospects. Gastroenterol Hepatol 27(Suppl 1):40–48
Romero-Gomez M (2005) Role of phosphate-activated glutaminase in the pathogenesis of hepatic encephalopathy. Metab Brain Dis 20:319–325
Romero-Gomez M, Grande L, Camacho I (2004a) Prognostic value of altered oral glutamine challenge in patients with minimal hepatic encephalopathy. Hepatology 39:939–943
Romero-Gomez M, Ramos-Guerrero R, Grande L, de Teran LC, Corpas R, Camacho I, Bautista JD (2004b) Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 41:49–54
Romero-Gómez M, Hoyas E, Viloria MM, Jover M, Córpas R, Collantes-de-Terán L, Camacho I, Cruz M, Bautista JD (2005) Oral glutamine challenge response is regulated by portal hypertension and systemic inflammatory response. J Hepatol 42(suppl 1):182A
Shawcross DL, Davies NA, Williams R, Jalan R (2004) Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 40:247–254
Sherlock S (1987) Chronic portal-systemic encephalopathy: update 1987. Gut 28:1043–1048
Taylor L, Liu X, Newsome W, Shapiro RA, Srinivasan M, Curthoys NP (2001) Isolation and characterization of the promoter region of the rat kidney-type glutaminase gene. Biochim Biophys Acta 1518:132–136
Warren KS, Newton WL (1959) Portal and peripheral blood Ammonia concentrations in germ-free and convencional guinea pigs. Am J Pysiol 197:717–720
Weber FJL, Veach GL (1979) The importance of the small intestine in gut ammonium production in the fasting dog. Gastroenterology 77:235–240
Wong D, Dorovini-Zis K, Vincent SR (2004) Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol 190:446–455
Wright G, Jalan R (2007) Ammonia and inflammation in the pathogenesis of hepatic encephalopathy: Pandora’s box? Hepatology 46:291–294
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero-Gómez, M., Jover, M., Galán, J.J. et al. Gut ammonia production and its modulation. Metab Brain Dis 24, 147–157 (2009). https://doi.org/10.1007/s11011-008-9124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-008-9124-3