Abstract
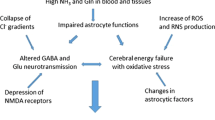
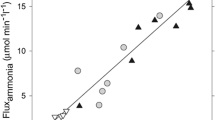
Results of neuropathologic, spectroscopic, and neurochemical studies continue to confirm a major role for ammonia in the pathogenesis of the central nervous system complications of both acute and chronic liver failure. Damage to astrocytes characterized by cell swelling (acute liver failure) or Alzheimer Type II astrocytosis (chronic liver failure) can be readily reproduced by acute or chronic exposure of these cells in vitro to pathophysiologically relevant concentrations of ammonia. Furthermore, exposure of the brain or cultured astrocytes to ammonia results in similar alterations in expression of genes coding for key astrocytic proteins. Such proteins include the structural glial fibrillary acidic protein, glutamate transporters, and peripheral-type (mitochondrial) benzodiazepine receptors. Brain–blood ammonia concentration ratios (normally of the order of 2) are increased up to fourfold in liver failure and arterial blood ammonia concentrations are good predictors of cerebral herniation in patients with acute liver failure. Studies using 1H magnetic resonance spectroscopy in patients with chronic liver failure reveal a positive correlation between the severity of neuropsychiatric symptoms and brain concentrations of the brain ammonia-detoxification product glutamine. Increased intracellular glutamine may be a contributory cause of brain edema in hyperammonemia. Positron emission tomography studies using 13HN3 provide evidence of increased blood–brain ammonia transfer and brain ammonia utilization rates in patients with chronic liver failure. In addition to the use of nonabsorbable disaccharides and antibiotics to reduce gut ammonia production, new approaches to the treatment of hepatic encephalopathy by lowering of brain ammonia include the use of L-ornithine–L-aspartate and mild hypothermia.
Similar content being viewed by others
REFERENCES
Bélanger, M., Desjardins, P., Chatauret, N., and Butterworth, R.F. (2002). Loss of expression of glial fibrillary acidic protein in acute hyperammonemia. Neurochem. Int. 41:155-160.
Butterworth, R.F. (1998). Alterations of neurotransmitter-related gene expression in human and experimental portal-systemic encephalopathy. Metab. Brain Dis. 13:339-347.
Butterworth, R.F., and Giguère, J.F. (1986). Cerebral amino acids in portal-systemic encephalopathy: Lack of evidence for altered GABA function. Metab. Brain Dis. 1:221-228.
Butterworth, R.F., Giguère, J.-F., Michaud, J., Lavoie, J., and Pomier Layrargues, G. (1987). Ammonia: Key factor in the pathogenesis of hepatic encephalopathy. Neurochem. Pathol. 6:1-12.
Chan, H., and Butterworth, R.F. (1999). Evidence for an astrocytic glutamate transporter deficit in hepatic encephalopathy. Neurochem. Res. 24:1397-1401.
Clemmensen, J.O., Larsen, F.S., Kondrup, J., Hansen, B.A., and Ott, P. (1999). Cerebral herniation in patients with acute liver is correlated with arterial ammonia concentration. Hepatology 29: 648-653.
Cooper, A.J.L., and Plum, F. (1987). Biochemistry and physiology of brain ammonia. Physiol. Rev. 67:40-519.
Dejong, C.H.C., Kampman, M.T., Deutz, N.E.P., and Soeters, P.B. (1992). Cerebral cortex ammonia and glutamine metabolism during liver insufficiency-induce hyperammonemia in the rat. J. Neurochem. 59:1071-1079.
Desjardins, P., Bandeira, P., Raghavendra Rao, V.K., and Butterworth, R.F. (1999). Portacaval anastomosis causes selective alterations of peripheral-type benzodiazepine receptor expression in rat brain and peripheral tissues. Neurochem Int. 35:293-299.
Gabuzda, G., Jr., Phillips, G.B., and Davidson, C.S. (1952). Reversible toxic manifestations in patients with cirrhosis of the liver given cation-exchange resins. N. Engl. J. Med. 246:124-130.
Gibson, G.E., Zimber, A., Krook, L., Richardson, E.P., Jr., and Visek, W.J. (1974). Brain histology and behaviour of mice injected with urease. J. Neuropathol. Exp. Neurol. 33:201-211.
Giguère, J.F., and Butterworth, R.F. (1984). Amino acid changes in regions of the CNS in relation to function in experimental portal-systemic encephalopathy. Neurochem. Res. 9:1309-1321.
Gregorios, J.B., Mozes, L.W., Norenberg, L.O., and Norenberg, M.D. (1985). Morphologic effects of ammonia on primary astrocyte cultures. I. Light microscopic studies. J. Neuropathol. Exp. Neurol. 44:397-403.
Hahn, M., Massen, O., Nenki, M., and Pavlov, J. (1893). Die Ecksche Fistel zwischen der unteren Hohlvene und der Pfortader und ihre Folger fur den Organismus. Arch. Exp. Pathol. Pharmakol. 32:161-270.
Harper, C.G., and Butterworth, R.F. (1997). Nutritional and metabolic disorders. In: Graham, D.I. and Lantos, P.L. (eds.), Greenfield's Neuropathology, Arnold, London, UK, pp. 601-655.
Hindfelt, B., Plum, F., and Duffy, T.E. (1977). Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J. Clin. Invest. 59:386-396.
Kato, M.D., Hughes, R.D., Keays, R.T., and Williams, R. (1992). Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 15: 1060-1066.
Kendall, B. E., Kingsley, D.P.E., Leonard, J.V., Lingam, S., and Oberholzer, V. G. (1983). Neurological features and computed tomography of the brain in children with ornithine carbamoyl transferase deficiency. J. Neurol., Neurosurg. Psychiatry 46:28-34.
Kircheis, G., Nilius, R., Held, C., Berndt, H., Buchner, M., Gortelmeyer, R., et al. (1997). Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: Results of a placebo-controlled, double-blind. Hepatology 25:1351-1360.
Laubenberger, J., Haussinger, D., Boyer, S., Guffer, H., Henning, J., and Lange, M. (1997). Proton magnetic resonance spectroscopy of brain in symptomatic and asymptomatic patients with liver cirrhosis. Gastroenterology 112:1610-1616.
Lavoie, J., Giguère, J.F., Pomier Layrargues, G., and Butterworth, R.F. (1987). Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J. Neurochem. 49: 692-697.
Lockwood, A.M., McDonald, J.M., and Rieman, R.E. (1979). The dynamics of ammonia metabolism in man: Effects of liver disease and hyperammonemia. J. Clin. Invest. 63:449-460.
Lockwood, A.H., Yap, E.W.H., and Wong, W.-H. (1991). Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J. Cereb. Blood Flow Metab. 11: 337-341.
Matthews, S.A. (1922). Ammonia, a causative factor in meat poisoning in Eck fistula dogs. Am. J. Physiol. 59:459-460.
Norenberg, M.D. (1988). Hepatic encephalopathy: Studies with astrocyte cultures. In: Norenberg, M.D., Hertz, L., Schousboe, A. (eds.), The Biochemical Pathology of Astrocytes, Alan R. Liss, New York, pp. 451-464.
Rose, C., Michalak, A., Pannunzio, P., Therrien, G., Quack, G., Kircheis, G., et al. (1998). Orthinine-L-Aspartate in experimental portal-systemic encephalopathy: Therapeutic efficacy and mechanism of action. Metab. Brain Dis. 13:147-157.
Swain, M., Butterworth, R.F., and Blei, A.T. (1992). Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 15:449-453.
Swart, G. R., Van den Berg, J.W., Wattimena, J.L., Rietveld, T., Von Vuure, J.K., and Frenkel, M. (1988). Elevated protein requirement in cirrhosis of the liver investigated by whole body protein turnover studies. Clin. Sci. (Lond) 75:101-107.
Therrien, G., Rose, C., and Butterworth, R.F. (1995). Early loss of day-night rhythms following portacaval anastomosis in the rat. In: Capocaccia, L., Merli, M., and Riggio, O. (eds.), Advances in Hepatic Encephalopathy and Metabolic Nitrogen Exchange, CRC Press, Boca Raton, FL, pp. 304-307.
Traber, P.G., DalCanto, M., Ganger, D.R., and Blei, A.T. (1987). Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: Ultrastructure and integrity of the blood-brain barrier. Hepatology 7:1272-1277.
Wolpert, E., Phillips, S.F., and Summerskill, W.H. (1970). Ammonia production in the human colon: Effects of cleansing, neomycin and acetohydroxamic acid. N. Engl. J. Med. 283:159-164.
Zieve, L., Doizaki, W.M., and Zieve, F. (1974). Synergism between mercaptans and ammonia or fatty acids in the production of coma: A possible role for mercaptans in the pathogenesis of hepatic coma. J. Lab. Clin. Med. 83:16-28.
Rights and permissions
About this article
Cite this article
Butterworth, R.F. Pathophysiology of Hepatic Encephalopathy: A New Look at Ammonia. Metab Brain Dis 17, 221–227 (2002). https://doi.org/10.1023/A:1021989230535
Issue Date:
DOI: https://doi.org/10.1023/A:1021989230535