Abstract
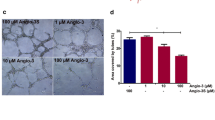
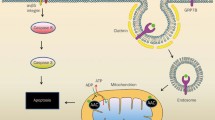
The study of angiogenesis, and the promise of angiogenesis inhibition as a means of cancer therapy, has dramatically accelerated in the last several years. The discovery and publication of angiostatin by O'Reilly and colleagues in Judah Folkman's lab in 1994 has greatly contributed to this progress. Angiostatin is a kringle-containing fragment of plasminogen, which is a potent inhibitor of angiogenesis in-vivo, and selectively inhibits endothelial cell proliferation and migration in-vitro. There have been a number of proposed proteolytic mechanisms by which plasminogen is cleaved to form angiostatin, and the resulting cleavage products contain different NH2 and COOH termini of the angiostatin. Therefore, it is possible that there are more than one angiostatin isoforms (or angiostatin-related proteins) which occur in one or more normal or pathophysiological situations. It is also possible that some of the proteolytic processes which can convert plasminogen to angiostatin-like proteins are simply laboratory artifacts. Angiostatin-related proteins exert potent endothelial cell inhibitory activity, including the induction of apoptosis, and inhibition of migration, and the intact kringle structures are believed to be necessary for the antiangiogenic activity. Efforts are now underway to translate the understanding of the biology of angiostatin to clinical practice, which includes phase 1 clinical trials with recombinant angiostatin K1–3 (kringles 1–3) as well as phase 1 trials of an Angiostatin Cocktail, which induces the direct in vivo conversion of plasminogen to angiostatin 4.5 (kringles 1–4, plus most of kringle 5). The translation of the basic science of angiostatin and angiostatin-related proteins to clinical trial promises to provide an important new tool in the treatment of cancer by inhibition of angiogenesis.
Similar content being viewed by others
References
Folkman J: Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182-6, 1971
O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J: Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma (see comments). Cell 79: 315-28, 1994
Machovich R, Owen W: An elastase-dependent pathway of plasminogen activation. Biochemistry 28: 4517-22, 1989
Gately S, Twardowski P, Stack MS, Patrick M, Boggio L, Cundiff DL, Schnaper HW, Madison L, Volpert O, Bouck N, Enghild J, Kwaan HC, Soff GA: Human prostate carcinoma cells express enzymatic activity that converts human plasminogen to the angiogenesis inhibitor, angiostatin. Cancer Res 56: 4887-90, 1996
Gately S, Twardowski P, Stack MS, Cundiff DL, Grella D, Castellino FJ, Enghild J, Kwaan HC, Lee F, Kramer RA, Volpert O, Bouck N, Soff GA: The mechanism of cancer-mediated conversion of plasminogen to the angiogenesis 106 inhibitor angiostatin. Proc Natl Acad Sci USA 94: 10 868-72, 1997
Falcone DJ, Khan KM, Layne T, Fernandes L: Macrophage formation of angiostatin during inflammation. A byproduct of the activation of plasminogen. J Biol Chem 273: 31 480-5, 1998
O'Mahony CA, Seidel A, Albo D, Chang H, Tuszynski GP, Berger DH: Angiostatin generation by human pancreatic cancer. J Surg Res 77: 55-8, 1998
Stathakis P, Fitzgerald M, Matthias LJ, Chesterman CN, Hogg PJ: Generation of angiostatin by reduction and proteolysis of plasmin. Catalysis by a plasmin reductase secreted by cultured cells. J Biol Chem 272: 20 641-5, 1997
Stathakis P, Lay AJ, Fitzgerald M, Schlieker C, Matthias LJ, Hogg PJ: Angiostatin formation involves disulfide bond reduction and proteolysis in kringle 5 of plasmin. J Biol Chem 274: 8910-6, 1999
Soff GA, Hong J, Fishman D, Schultz R, Cundiff D, Park S, Enghild J, Stack MS, Gately S: Angiostatin4.5: a naturally occurring human angiogenesis inhibitor. Proc Am Assoc Canc Res 40: 4088, 1999
Dong Z, Kumar R, Yang X, Fidler IJ: Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 88: 801-10, 1997
Patterson BC, Sang QA: Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). J Biol Chem 272: 28 823-5, 1997
Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD: Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol 161: 6845-52, 1998
Lijnen HR, Ugwu F, Bini A, Collen D: Generation of an angiostatin-like fragment from plasminogen by stromelysin-1 (MMP-3). Biochemistry 37: 4699-702, 1998
Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O'Reilly M, Folkman J: Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc Natl Acad Sci USA 95: 5579-83, 1998
Lucas R, Holmgren L, Garcia I, Jimenez B, Mandriota SJ, Borlat F, Sim BK, Wu Z, Grau GE, Shing Y, Soff GA, Bouck N, Pepper MS: Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood 92: 4730-41, 1998
Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV: Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA 96: 2811-6, 1999
Redlitz A, Daum G, Sage EH: Angiostatin diminishes activation of the mitogen-activated protein kinases ERK-l and ERK-2 in human dermal microvascular endothelial cells. J Vasc Res 36: 28-34, 1999
Stack MS, Gately S, Bafetti LM, Enghild JJ, Soff GA: Angiostatin inhibits endothelial and melanoma cellular invasion by blocking matrix-enhanced plasminogen activation. Biochem J 340: 77-84, 1999
Bachman F: The plasminogen-plasmin enzyme system. In: Colman RW, Hirsh J, Marder V, Salzman EW (eds), Hemostasis and Thrombosis: Basic Principles and Clinical Practice, Vol. 3, Philadelphia: J.B. Lippincott, 1994, pp 1592-1622
Sim BK, O'Reilly MS, Liang H, Fortier AH, He W, Madsen JW, Lapcevich R, Nacy CA: A recombinant human angiostatin protein inhibits experimental primary and metastatic cancer. Cancer Res 57: 1329-34, 1997
Cao Y, Ji RW, Davidson D, Schaller J, Marti D, Sohndel S, McCance SG, O'Reilly MS, Llinas M, Folkman J: Kringle domains of human angiostatin. Characterization of the antiproliferative activity on endothelial cells. J Biol Chem 271: 29 461-7, 1996
Lee TH, Rhim T, Kim SS: Prothrombin kringle-2 domain has a growth inhibitory activity against basic fibroblast growth factor-stimulated capillary endothelial cells. J Biol Chem 273: 28 805-12, 1998
Cao Y, Chen A, An SSA, Ji RW, Davidson D, Llinas M: Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J Biol Chem 272: 22 924-8, 1997
Ji WR, Castellino FJ, Chang Y, Deford ME, Gray H, Villarreal X, Kondri ME, Marti DN, Llinas M, Schaller J, Kramer RA, Trail PA: Characterization of kringle domains of angiostatin as antagonists of endothelial cell migration, an important process in angiogenesis. Faseb J 12: 1731-8, 1998
Cao Y, Veitonmaki N, Keough K, Cheng H, Lee LS, Zurakowski D: Elevated levels of urine angiostatin and plasminogen/ plasmin in cancer patients (in process citation). Int J Mol Med 5: 547-51, 2000
Sack RA, Beaton AR, Sathe S: Diurnal variations in angiostatin in human tear fluid: a possible role in prevention of corneal neovascularization. Curr Eye Res 18: 186-93, 1999
MacDonald NJ, Murad AC, Fogler WE, Lu Y, Sim BK: The tumor-suppressing activity of angiostatin protein resides within kringles 1 to 3. Biochem Biophys Res Commun 264: 469-77, 1999
Meneses PI, Abrey LE, Hajjar KA, Gultekin SH, Duvoisin RM, Berns KI, Rosenfeld MR: Simplified production of a recombinant human angiostatin derivative that suppresses intracerebral glial tumor growth. Clin Cancer Res 5: 3689-94, 1999
Nguyen JT, Wu P, Clouse ME, Hlatky L, Terwilliger EF: Adeno-associated virus-mediated delivery of antiangiogenic factors as an antitumor strategy. Cancer Res 58: 5673-7, 1998
Cao Y, O'Reilly MS, Marshall B, Flynn E, Ji RW, Folkman J: Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases (published erratum appears in J Clin Invest 102(11): 2031 1 Dec 1998). J Clin Invest 101: 1055-63, 1998
Tanaka T, Cao Y, Folkman J, Fine HA: Viral vector-targeted antiangiogenic gene therapy utilizing an angiostatin complementary DNA. Cancer Res 58: 3362-9, 1998
Ambs S, Dennis S, Fairman J, Wright M, Papkoff J: Inhibition of tumor growth correlates with the expression level of a human angiostatin transgene in transfected B16F10 melanoma cells. Cancer Res 59: 5773-7, 1999
Chen QR, Kumar D, Stass SA, Mixson AJ: Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res 59: 3308-12, 1999
Teicher BA, Holden SA, Ara G, Northey D: Response of the FSaII fibrosarcoma to antiangiogenic modulators plus cytotoxic agents. Anticancer Res 13: 2101-6, 1993
Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M, Soff GA, Sukhatme VP, Kufe DW, Weichselbaum RR: Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature 394: 287-91, 1998
Gorski DH, Mauceri HJ, Salloum RM, Gately S, Hellman S, Beckett MA, Sukhatme VP, Soff GA, Kufe DW, Weichselbaum RR: Potentiation of the antitumor effect of ionizing radiation by brief concomitant exposures to angiostatin. Cancer Res 58: 5686-9, 1998
Soff GA, Rossbach HC, Hoppin EC, Cundiff D, Schultz R, Kunz P: Therapeutic application of an angiostatic cocktail for patients with refractory cancer. Proc Am Assoc Canc Res 41: 1960, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soff, G.A. Angiostatin and Angiostatin-related Proteins. Cancer Metastasis Rev 19, 97–107 (2000). https://doi.org/10.1023/A:1026525121027
Issue Date:
DOI: https://doi.org/10.1023/A:1026525121027