Abstract
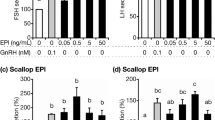
Inhibin is a gonadal protein that specifically inhibits the secretion of pituitary follicle-stimulating hormone (FSH). Two forms of inhibin (A and B) have been purified from porcine follicular fluid1 and characterized as heterodimers of relative molecular mass (Mr) 32,000 (ref. 2). Each inhibin is comprised of an identical α-subunit of Mr 18,000 and a distinct but related β-subunit of Mr 13,800–14,700 linked by interchain disulphide bond(s). Throughout the purification of inhibins, we consistently observed two fractions which stimulated the secretion of pituitary FSH. We report here the isolation of one of the FSH-releasing proteins; it has a Mr of 24,000 and its N-terminal sequences up to residue 32 are identical to those of each β-subunit of inhibins A and B. In the presence of reducing agents, SDS-polyacrylamide gel electrophoresis resolves the FSH-releasing substance into two subunits which are identical in their migration behaviour to the reduced β-subunits of inhibins A and B. Based on the N-terminal sequence data and Mr of the intact and reduced molecules, we propose that the FSH-releasing substance, which is active in picomolar concentrations, is a heterodimeric protein composed of the two β-subunits of inhibins A and B linked by interchain disulphide bond(s). The structural organization of the FSH-releasing substance is homologous to that of transforming growth factor-β (TGF-β)3, which also possesses FSH-releasing activity in the same bioassay4. We suggest that the substance be called activin to signify the fact that it has opposite biological effects to inhibin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ling, N. et al. Proc. natn. Acad. Sci. U.S.A. 82, 7217–7221 (1985).
Mason, A. J. et al. Nature 318, 659–663 (1985).
Derynck, R. et al. Nature 316, 701–705 (1985).
Ying, S-Y. et al. Biochem. biophys. Res. Commun. 135, 950–956 (1986).
Docherty, K. & Steiner, D. F. A. Rev. Physiol. 44, 625–638 (1982).
Waterfield, M. D. et al. Nature 304, 35–39 (1983).
Heldin, C. H. et al. Nature 319, 511–514 (1986).
Laemmli, U. Nature 227, 680–685 (1970).
Esch, F. Analyt. Biochem. 136, 39–47 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ling, N., Ying, SY., Ueno, N. et al. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 321, 779–782 (1986). https://doi.org/10.1038/321779a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/321779a0
This article is cited by
-
Fibronectin mediates activin A-promoted human trophoblast migration and acquisition of endothelial-like phenotype
Cell Communication and Signaling (2024)
-
Effect of maternal polycystic ovary syndrome (PCOS) on screening of aneuploidy in the first and second trimesters
Journal of Ovarian Research (2023)
-
Diabetes as a potential compounding factor in COVID-19-mediated male subfertility
Cell & Bioscience (2022)
-
Mitochondrial regulation during male germ cell development
Cellular and Molecular Life Sciences (2022)
-
Insights into in vivo follicle formation: a review of in vitro systems
Histochemistry and Cell Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.