Abstract
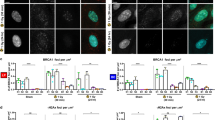
Much of the predisposition to hereditary breast and ovarian cancer has been attributed to inherited defects in the BRCA1 tumour-suppressor gene1,2,3. The nuclear protein BRCA1 has the properties of a transcription factor4,5,6,7, and can interact with the recombination and repair protein RAD51 (ref. 8). Young women with germline alterations in BRCA1 develop breast cancer at rates 100-fold higher than the general population3, and BRCA1-null mice die before day 8 of development9,10. However, the mechanisms of BRCA1-mediated growth regulation and tumour suppression remain unknown. Here we show that BRCA1 transactivates expression of the cyclin-dependent kinase inhibitor p21WAF1/CIP1 in a p53-independent manner, and that BRCA1 inhibits cell-cycle progression into the S-phase following its transfection into human cancer cells. BRCA1 does not inhibit S-phase progression in p21−/− cells, unlike p21+/+ cells, and tumour-associated, transactivation-deficient mutants of BRCA1 are defective in both transactivation of p21 and cell-cycle inhibition. These data suggest that one mechanism by which BRCA1 contributes to cell-cycle arrest and growth suppression is through the induction of p21.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Miki, Y. et al. Astrong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Futreal, P. A. et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science 266, 120–122 (1994).
Easton, D. F., Ford, D. & Bishop, D. T. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Am. J. Hum. Genet. 56, 265–271 (1995).
Chen, Y. et al. Aberrant subcellular localization of BRCA1 in breast cancer. Science 270, 789–791 (1995).
Scully, R. et al. Location of BRCA1 in human breast and ovarian cancer cells. Science 272, 123–125 (1996).
Chapman, M. S. & Verma, I. M. Transcriptional activation by BRCA1. Nature 382, 678–679 (1996).
Monteiro, A. N., August, A. & Hanafusa, H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc. Natl Acad. Sci. USA 93, 13595–13599 (1996).
Scully, R. et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88, 265–275 (1997).
Gowen, L. C. et al. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nature Genet. 12, 191–194 (1996).
Hakem, R. et al. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell 85, 1009–1023 (1996).
Sherr, C. J. & Roberts, J. M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9, 1149–1163 (1995).
Zeng, Y.-X., Somasundaram, K., Prabhu, N. S., Krishnadasan, R. & El-Deiry, W. S. Detection and analysis of living, growth-inhibited mammalian cells following transfection. Biotechniques 23, 88–94 (1997).
El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Zhang, W. et al. p53-independent induction of WAF1/CIP1 in human leukeumia cells is correlated with growth arrest accompanying monocyte/macrophage differentiation. Cancer Res. 55, 668–674 (1995).
Waldman, T., Kinzler, K. W. & Vogelstein, B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 55, 5187–5190 (1995).
Vaughn, J. P. et al. BRCA1 expression is induced before DNA synthesis in both normal and tumor-derived breast cells. Cell Growth Differ. 7, 711–715 (1996).
Gudas, J. M. et al. Cell cycle regulation of BRCA1 messenger RNA in human breast epithelial cells. Cell Growth Differ. 7, 717–723 (1996).
Chen, Y. et al. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 56, 3168–3172 (1996).
Humphrey, J. S. et al. Human BRCA1 inhibits growth in yeast: Potential use in diagnostic testing. Proc. Natl Acad. Sci. USA 94, 5820–5825 (1997).
Scully, R. et al. BRCA1 is a component of the RNA polymerase II holoenzyme. Proc. Natl Acad. Sci. USA 94, 5605–5610 (1997).
Zeng, Y.-X., Somasundaram, K. & El-Deiry, W. S. AP2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nature Genet. 15, 78–82 (1997).
El-Deiry, W. S. et al. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 55, 2910–2919 (1995).
Thakur, S. et al. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol. Cell. Biol. 17, 444–452 (1997).
Meyuhas, O. & Perry, R. P. Construction and identification of cDNA clones for mouse ribosomal proteins: application for the study of r-protein gene expression. Gene 10, 113–129 (1980).
Somasundaram, K. & El-Deiry, W. S. Inhibition of p53-mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene 14, 1047–1057 (1997).
Acknowledgements
This work was supported by grants from the NIH and the Breast Cancer Research Foundation to B.L.W. W.S.E.-D. is an assistant investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Somasundaram, K., Zhang, H., Zeng, YX. et al. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiPl. Nature 389, 187–190 (1997). https://doi.org/10.1038/38291
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/38291
This article is cited by
-
Induction of cell cycle arrest and apoptosis by CPUC002 through stabilization of p53 and suppression of STAT3 signaling pathway in multiple myeloma
Cell Biology and Toxicology (2021)
-
Expression of tripartite motif-containing 44 and its prognostic and clinicopathological value in human malignancies:a meta-analysis
BMC Cancer (2020)
-
RBBP8/CtIP suppresses P21 expression by interacting with CtBP and BRCA1 in gastric cancer
Oncogene (2020)
-
TRIM44 is indispensable for glioma cell proliferation and cell cycle progression through AKT/p21/p27 signaling pathway
Journal of Neuro-Oncology (2019)
-
Trans-activation-based risk assessment of BRCA1 BRCT variants with unknown clinical significance
Human Genomics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.