Abstract
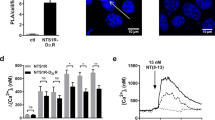
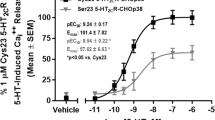
The neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) elicits a wide array of physiological effects by binding to several receptor subtypes. The 5-HT2 family of receptors belongs to a large group of seven-transmembrane-spanning G-protein-coupled receptors and includes three receptor subtypes (5-HT2A, 5-HT2B and 5-HT2C) which are linked to phospholipase C, promoting the hydrolysis of membrane phospholipids and a subsequent increase in the intracellular levels of inositol phosphates and diacylglycerol1. Here we show that transcripts encoding the 2C subtype of serotonin receptor (5-HT2CR) undergo RNA editing events in which genomically encoded adenosine residues are converted to inosines by the action of double-stranded RNA adenosine deaminase(s). Sequence analysis of complementary DNA isolates from dissected brain regions have indicated the tissue-specific expression of seven major 5-HT2C receptor iso-forms encoded by eleven distinct RNA species. Editing of 5-HT2CR messenger RNAs alters the amino-acid coding potential of the predicted second intracellular loop of the receptor and can lead to a 10–15-fold reduction in the efficacy of the interaction between receptors and their G proteins. These observations indicate that RNA editing is a new mechanism for regulating serotonergic signal transduction and suggest that this post-transcriptional modification may be critical for modulating the different cellular functions that are mediated by other members of the G-protein-coupled receptor superfamily.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hoyer, D. et al. IUP classification of receptors for 5-hydroxytryptamine (serotonin). Pharrnacol. Rev. 46, 157–203 (1994).
Polson, A. G., Bass, B. L. & Casey, J. L. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature 380, 454–456 (1996).
Sommer, B., Kohler, M., Sprengel, R. & Seeburg, P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19 (1991).
Lomeli, H. et al. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 266, 1709–1713 (1994).
Kohler, M., Burnashev, N., Sakmann, B. & Seeburg, P. Determinants of Ca2+ permeabiity in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron 10, 491–500 (1993).
Egebjerg, J. & Heinemann, S. F. Intron sequence directs RNA editing of the glutamate receptor subunit GluR2 coding sequence. Proc. Natl Acad. Sci. USA 90, 755–759 (1993).
Zuker, M. Computer prediciton of RNA structure. Meth. Enzymol. 180, 262–288 (1989).
Higuchi, M. et al. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines positon and efficiency. Cell 75, 1361–1370 (1993).
Herb, A., Higuchi, M., Sprengel, R. & Seeburg, P. H. Q/R site editing in kainate receptor GluRS and GluR6 pre-mRNAs requires distant intronic sequences. Proc. Natl Acad. Sci. USA 93, 1875–1880 (1996).
Rueter, S. et al. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science 267, 1491–1494 (1995).
Yang, J.H., Sklar, P., Axel, R. & Maniatis, T. Editing of glutamate receptor subunit B pre-mRNA in vitro by site-specific deamination of adenosine. Nature 374, 77–81 (1995).
Maas, S. et al. Structural requirements for RNA editing in glutamate receptor pre-mRNAs by recombinant double-stranded RNA adenosine deaminase. J. Biol. Chem. 271, 12221–12226 (1996).
Melcher, T. et al. A mammalian RNA editing enzyme. Nature 379, 460–464 (1996).
Bass, B. RNA editing: New uses for old players in the RNA world. The RNA World 383–418 (Cold Spring Harbor Laboratory Press, New York, 1993).
Gomeza, J. et al. The second intracellular loop of metabotropic glutamate receptor 1 cooperates with the other intracellular domains to control coupling to G-proteins. J. Biol. Chem. 271, 2199–2205 (1996).
Westphal, R. S., Backstrom, J. R. & Sanders-Bush, E. Increased basal phosphorylation of the constitutively active serotonin 2C receptor accompanies agonist-mediated desensitization. Mol. Pharmacol. 48, 200–205 (1995).
Ariëns, E. J., Beld, A. J., Rodrigues de Miranda, J. F. & Simonis, A. M. The Receptors 33–91 (Plenum, New York, 1979).
Meller, E. et al. Receptor reserve for D2 dopaminergic inhibition of prolactin release in vivo and in vitro. J. Pharmacol. Exp. Ther. 257, 668–675 (1991).
Leonhardt, S., Garospe, E.,, Hoffman, B. J. & Teitler, M. Molecular pharmacological differences in the interaction of serotonin with 5-hydroxytryptamine 1C and 5-hydroxytryptamine2 receptors. Mol. Pharmacol. 42, 328–335 (1992).
Ross, E. M. G protein GTPase-activating proteins: regulation of speed, amplitude, and signaling selectivity. Rec. Prog. Harm. Res. 50, 207–221 (1995).
Moro, O., Lameh, J., Högger, P. & Sadée, W. Hydrophobic amino acid in the 12 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 268, 22273–22276 (1993).
Yu, L. et al. The mouse 5-HT1C receptor contains eight hydrophobic domains and is X-linked. Mol. Brain Res. 11, 143–149 (1991).
Kennelly, P. J. & Krebs, E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 266, 15555–15558 (1991).
Julius, D., MacDermott, A. B., Axel, R. & Jessell, T. M. Molecular characterization of a functional cDNA encoding the serotonin Ic receptor. Science 241, 558–264 (1988).
Ausubel, F. et al. (eds) Current Protocols in Molecular Biology (Wiley, New York, 1989).
O'Connell, M. et al. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol. Cell Biol. 15, 1389–1397 (1995).
Gorski, K., Carneiro, M. & Schibler, U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell 47, 767–776 (1986).
Barker, E. L., Westphal, R. S., Schmidt, D. & Sanders-Bush, E. Constitutively active 5-hydroxytrypta-mine2C receptors reveal novel inverse agonist activity of receptor ligands. J. Biol Chem. 269, 11687–11690 (1994).
Westphal, R. S. & Sanders-Bush, E. Reciprocal binding properties of 5-hydroxytryptamine type 2C receptor agonists and inverse agonists. Mol. Pharmacol. 46, 937–942 (1994).
Cheng, Y. & Prusoff, W. H. Relationship between the inhibiton constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burns, C., Chu, H., Rueter, S. et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387, 303–308 (1997). https://doi.org/10.1038/387303a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387303a0
This article is cited by
-
Deep transcriptome profiling reveals limited conservation of A-to-I RNA editing in Xenopus
BMC Biology (2023)
-
The cellular and KSHV A-to-I RNA editome in primary effusion lymphoma and its role in the viral lifecycle
Nature Communications (2023)
-
Dissecting the basis for differential substrate specificity of ADAR1 and ADAR2
Nature Communications (2023)
-
Ethanol deprivation and central 5-HT deficiency differentially affect the mRNA editing of the 5-HT2C receptor in the mouse brain
Pharmacological Reports (2023)
-
Endogenous ADAR-mediated RNA editing in non-human primates using stereopure chemically modified oligonucleotides
Nature Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.