Abstract
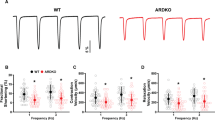
The sympathetic nervous system regulates cardiovascular function by activating adrenergic receptors in the heart, blood vessels and kidney1. α2-Adrenergic receptors are known to have a critical role in regulating neurotransmitter release from sympathetic nerves and from adrenergic neurons in the central nervous system2,3,4,5; however, the individual roles of the three highly homologous α2-adrenergic-receptor subtypes (α2A, α2B, α2C) in this process are not known. We have now studied neurotransmitter release in mice in which the genes encoding the three α2-adrenergic-receptor subtypes were disrupted. Here we show that both the α2A- and α2C-subtypes are required for normal presynaptic control of transmitter release from sympathetic nerves in the heart and from central noradrenergic neurons. α2A-Adrenergic receptors inhibit transmitter release at high stimulation frequencies, whereas the α2C-subtype modulates neurotransmission at lower levels of nerve activity. Both low- and high-frequency regulation seem to be physiologically important, as mice lacking both α2A- and α2C-receptor subtypes have elevated plasma noradrenaline concentrations and develop cardiac hypertrophy with decreased left ventricular contractility by four months of age.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Taylor,A. A. Autonomic control of cardiovascular function: clinical evaluation in health and disease. J. Clin. Pharmacol. 34, 363–374 (1994).
Miller,R. J. Presynaptic recetors. Annu. Rev. Pharmacol. Toxicol. 38, 201–227 (1998).
Langer,S. Z. 25 years since the discovery of presynaptic recetors: present knowledge and future perspectives. Trends Pharmacol. Sci. 18, 95–99 (1997).
Starke,K., Göthert,M. & Kilbinger,H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 69, 864–989 (1989).
Stjärne,L. Basic mechanisms and local modulation of nerve impulse-induced secretion of neurotransmitters from individual sympathetic nerve varicosities. Rev. Physiol. Biochem. Pharmacol. 112, 4–137 (1989).
Bylund,D. B. et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 46, 121–136 (1994).
Link,R. E. et al. Targeted inactivation of the gene encoding the mouse α2C-adrenoceptor homolog. Molec. Pharmacol. 48, 48–55 (1995).
Link,R. E. et al. Cardiovascular regulation in mice lacking α2-adrenergic receptor subtypes b and c. Science 273, 803–805 (1996).
MacMillan,L. B., Hein,L., Smith,M. S., Piascik,M. T. & Limbird,L. E. Central hypotensive effects of the α2A-adrenergic receptor subtype. Science 273, 801–803 (1996).
Sallinen,J. et al. Genetic alteration of α2C-adrenoceptor expression in mice: influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a subtype-nonselective α2-adrenoceptor agonist. Molec. Pharmacol. 51, 36–46 (1997).
Sallinen,J., Haapalinna,A., Vitamaa,T., Kobilka,B. K. & Scheinin,M. Adrenergic α2C-receptors modulate the acoustic startle reflex, prepulse inhibition, and aggression in mice. J. Neurosci. 18, 3035–3042 (1998).
Altman,J. D. et al. Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Molec. Pharmacol. 56, 154–161 (1999).
Singer,E. A. Transmitter release from brain slices elicited by single pulses: a powerful method to study presynaptic mechanisms. Trends Pharmacol. Sci. 9, 274–276 (1988).
Wahl,C. A., Trendelenburg,A. U. & Starke,K. Presynaptic α2-autoreceptors in mouse heart atria: evidence for the α2D subtype. Naunyn-Schmiedeberg's Arch. Pharmacol. 354, 253–261 (1996).
Trendelenburg,A. U., Limberger,N. & Starke,K. Presynaptic α2-autoreceptors in brain cortex: α2D in the rat and α2A in the rabbit. Naunyn-Schmiedeberg's Arch. Pharmacol. 348, 35–45 (1993).
Trendelenburg,A. U. et al. A re-investigation of questionable subclassifications of presynaptic α2-autoreceptors: rat vena cava, rat atria, human kidney and guinea-pig urethra. Naunyn-Schmiedeberg's Arch. Pharmacol. 356, 721–737 (1997).
Molderings,G. J. & Göthert,M. Subtype determination of presynaptic α2-autoreceptors in the rabbit pulmonary artery and human saphenous vein. Naunyn-Schmiedeberg's Arch. Pharmacol. 352, 483–490 (1995).
Smith,K., Gavin,K. & Docherty,J. R. Investigation of the subtype of α2-adrenoceptor mediating prejunctional inhibition of cardioacceleration in the pithed rat heart. Br. J. Pharmacol. 115, 316–320 (1995).
Link,R. E., Daunt,D., Barsh,G., Chruscinski,A. & Kobilka,B. Cloning of two mouse genes encoding α2-adrenergic receptor subtypes and identification of a single amino acid in the mouse α2-C10 homolog responsible for an interspecies variation in antagonist binding. Molec. Pharmacol. 42, 16–27 (1992).
McLachlan,E. M., Davies,P. J., Habler,H. J. & Jamieson,J. On-going and reflex synaptic events in rat superior cervical ganglion cells. J. Physiol (Lond.) 501, 165–181 (1997).
Lakhlani,P. P. et al. Substitution of a mutant α2A-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc. Natl Acad. Sci. USA 94, 9950–9955 (1997).
Packer,M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J. Am. Coll. Cardiol. 20, 248–254 (1992).
Cohn,J. N. et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984).
Lechat,P. et al. Clinical effects of β-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation 98, 1184–1191 (1998).
Hein,L., Barsh,G. S., Pratt,R. E., Dzau,V. J. & Kobilka,B. K. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature 377, 744–747 (1995).
Limberger,N., Trendelenburg,A. A. & Starke,K. Subclassification of presynaptic α2-adrenoceptors: α2D-autoreceptors in mouse brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 352, 43–48 (1995).
Taube,H. D., Starke,K. & Borowski,E. Presynaptic receptor systems on the noradrenergic neurones of rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 299, 123–141 (1977).
Graefe,K. H. et al. 1,1′-Diisopropyl-2,4′-cyanine (disprocynium24), a potent uptake2 blocker, inhibits the renal excretion of catecholamines. Naunyn-Schmiedeberg's Arch. Pharmacol. 356, 115–125 (1997).
Engelhardt,S., Hein,L., Wiesmann,F. & Lohse,M. J. progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl Acad. Sci. USA 96, 7059–7064 (1999).
Hein,L. et al. Overexpression of angiotensin AT1 receptor transgene in the mouse myocardium produces a lethal phenotype associated with myocyte hyperplasia and heart block. Proc. Natl Acad. Sci. USA 94, 6391–6396 (1997).
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft SFB355 (to L.H.) and the Howard Hughes Medical Institute (to B.K.). We thank K. Starke, A. U. Trendelenburg, M. J. Lohse and M. M. Bücheler) for consultation and assistance with transmitter release experiments; S. Engelhardt for assistance with the pathological analysis; R. Link for help with construction of the α2A-targeting vector; A. Chruscinski for help in characterizing the α2AC-KO mice; and K. H. Graefe and R. Wölfel for HPLC analysis of plasma noradrenaline levels.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hein, L., Altman, J. & Kobilka, B. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature 402, 181–184 (1999). https://doi.org/10.1038/46040
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/46040
This article is cited by
-
Dysregulation of hypoxia-inducible factor 1α in the sympathetic nervous system accelerates diabetic cardiomyopathy
Cardiovascular Diabetology (2023)
-
Methylation analysis by targeted bisulfite sequencing in large for gestational age (LGA) newborns: the LARGAN cohort
Clinical Epigenetics (2023)
-
Noradrenaline depresses facial stimulation-evoked cerebellar MLI-PC synaptic transmission via α2-AR/PKA signaling cascade in vivo in mice
Scientific Reports (2023)
-
Measuring neuron-regulated immune cell physiology via the alpha-2 adrenergic receptor in an ex vivo murine spleen model
Cellular and Molecular Life Sciences (2023)
-
Norepinephrine transporter defects lead to sympathetic hyperactivity in Familial Dysautonomia models
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.