Abstract
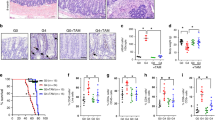
Telomerase activation is a common feature of advanced human cancers1 and facilitates the malignant transformation of cultured human cells2 and in mice3,4. These experimental observations are in accord with the presence of robust telomerase activity in more advanced stages of human colorectal carcinogenesis5,6,7. However, the occurrence of colon carcinomas in telomerase RNA (Terc)-null, p53-mutant mice8 has revealed complex interactions between telomere dynamics, checkpoint responses and carcinogenesis9. We therefore sought to determine whether telomere dysfunction exerts differential effects on cancer initiation versus progression of mouse and human intestinal neoplasia. In successive generations of ApcMin Terc−/− mice10,11, progressive telomere dysfunction led to an increase in initiated lesions (microscopic adenomas), yet a significant decline in the multiplicity and size of macroscopic adenomas. That telomere dysfunction also contributes to human colorectal carcinogenesis is supported by the appearance of anaphase bridges (a correlate of telomere dysfunction) at the adenoma-early carcinoma transition, a transition recognized for marked chromosomal instability12,13,14,15. Together, these data are consistent with a model in which telomere dysfunction promotes the chromosomal instability that drives early carcinogenesis, while telomerase activation restores genomic stability to a level permissive for tumor progression. We propose that early and transient telomere dysfunction is a major mechanism underlying chromosomal instability of human cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kim, N.W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2005 (1994).
Hahn, W.C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999).
Greenberg, R.A. et al. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell 97, 515–525 (1999).
Gonzalez-Suarez, E., Samper, E., Flores, J.M. & Blasco, M.A. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nature Genet . 26, 114–117 (2000).
Engelhardt, M., Drullinsky, P., Guillem, J. & Moore, MA. Telomerase and telomere length in the development and progression of premalignant lesions to colorectal cancer. Clin. Cancer Res . 3, 1931–1941 (1997).
Tang, R., Cheng, A.J., Wang, J.Y. & Wang, T.C. Close correlation between telomerase expression and adenomatous polyp progression in multistep colorectal carcinogenesis. Cancer Res. 58, 4052–4054 (1998).
Chadeneau, C., Hay, K., Hirte, H.W., Gallinger, S. & Bacchetti, S. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res. 55, 2533–2536 (1995).
Artandi, S. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000).
Hanahan, D. Benefits of bad telomeres. Nature 406, 573–574 (2000).
Moser, A.R., Pitot, H.C. & Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Su, L.K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Young, J. et al. Genomic instability occurs in colorectal carcinomas but not in adenomas. Hum. Mutat. 2, 351–354 (1993).
Okamoto, M. et al. Loss of constitutional heterozygosity in colon carcinoma from patients with familial polyposis coli. Nature 331, 273–277 (1988).
Ried, T. et al. Comparative genomic hybridization reveals a specific pattern of chromosomal gains and losses during the genesis of colorectal tumors. Genes Chromosom. Cancer 15, 234–245 (1996).
Meijer, G.A. et al. Progression from colorectal adenoma to carcinoma is associated with non-random chromosomal gains as detected by comparative genomic hybridisation. J. Clin. Pathol. 51, 901–909 (1998).
Lengauer, C., Kinzler, K.W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Dietrich, W.F. et al. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell 75, 631–639 (1993).
Halberg, R.B. et al. Tumorigenesis in the multiple intestinal neoplasia mouse: redundancy of negative regulators and specificity of modifiers. Proc. Natl. Acad. Sci. USA 97, 3461–3466 (2000).
Blasco, M.A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Herrera, E. et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999).
Lee, H.W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Rudolph, K.L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Luongo, C. & Dove, W.F. Somatic genetic events linked to the Apc locus in intestinal adenomas of the Min mouse. Genes Chromosom. Cancer 17, 194–198 (1996).
Luo, G. et al. Cancer predisposition caused by elevated mitotic recombination in bloom mice. Nature Genet. 26, 424–429 (2000).
McClintock, B. The stability of broken ends of chromosomes in Zea mays. Genetics 26, 234–282 (1941).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998).
Kirk, K.E., Harmon, B.P., Reichardt, I.K., Sedat, J.W. & Blackburn, E.H. Block in anaphase chromosome separation caused by a telomerase template mutation. Science 275, 1478–1481 (1997).
Rudolph, K.L., Chang, S., Millard, M., Schreiber-Agus, N. & DePinho, R.A. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287, 1253–1258 (2000).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Karlseder, J., Broccoli, D., Dai, Y., Hardy, S. & de Lange, T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283, 1321–1325 (1999).
Hastie, N.D. et al. Telomere reduction in human colorectal carcinoma and with ageing. Nature 346, 866–868 (1990).
Acknowledgements
We thank D. Castrillon and S. Chang for helpful advice regarding the pathological and histological classification of intestinal neoplasia; L. Chin, S. Weiler and R. Greenberg for critical review of the manuscript; and P. Flemming and M. Manns for access to the histology archives of the Medical School Hannover. K.L.R. was supported by Deutsche Forschungsgemeinschaft grant Ru 745/1-1, M.W.B. is supported by a Howard Hughes Medical Institute Physician Postdoctoral fellowship and the work was supported by National Institutes of Health grants to R.A.D., who is an American Cancer Society Research Professor and a Kirsch Foundation Scholar.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Rudolph, K., Millard, M., Bosenberg, M. et al. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 28, 155–159 (2001). https://doi.org/10.1038/88871
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/88871
This article is cited by
-
Targeting the “hallmarks of aging” to slow aging and treat age-related disease: fact or fiction?
Molecular Psychiatry (2023)
-
Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies
European Journal of Epidemiology (2021)
-
Aneuploidy-inducing gene knockdowns overlap with cancer mutations and identify Orp3 as a B-cell lymphoma suppressor
Oncogene (2020)
-
Building bridges between chromosomes: novel insights into the abscission checkpoint
Cellular and Molecular Life Sciences (2019)
-
Telomere heterogeneity linked to metabolism and pluripotency state revealed by simultaneous analysis of telomere length and RNA-seq in the same human embryonic stem cell
BMC Biology (2017)