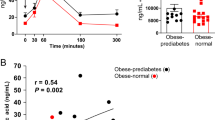
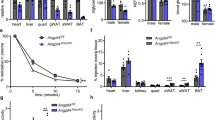
Abstract
Acrp30 is a circulating protein synthesized in adipose tissue. A single injection in mice of purified recombinant Acrp30 leads to a 2–3-fold elevation in circulating Acrp30 levels, which triggers a transient decrease in basal glucose levels. Similar treatment in ob/ob, NOD (non-obese diabetic) or streptozotocin-treated mice transiently abolishes hyperglycemia. This effect on glucose is not associated with an increase in insulin levels. Moreover, in isolated hepatocytes, Acrp30 increases the ability of sub-physiological levels of insulin to suppress glucose production. We thus propose that Acrp30 is a potent insulin enhancer linking adipose tissue and whole-body glucose metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mohamed-Ali, V., Pinkney, J.H. & Coppack, S.W. Adipose tissue as an endocrine and paracrine organ. Int. J. Obes. Relat. Metab. Disord. 22, 1145–1158 (1998).
Havel, P.J. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 59, 359–371 (2000).
Ahima, R.S. & Flier, J.S. Adipose Tissue as an Endocrine Organ. Trends Endocrinol. Metab. 11, 327–332 (2000).
Kahn, B.B. & Flier, J.S. Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 (2000).
Ganda, O.P. Lipoatrophy, lipodystrophy, and insulin resistance. Ann. Intern. Med. 133, 304–306 (2000).
Shimomura, I., Hammer, R.E., Ikemoto, S., Brown, M.S. & Goldstein, J.L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76 (1999).
Gavrilova, O. et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J. Clin. Invest. 105, 271–278 (2000).
Scherer, P.E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Hu, E., Liang, P. & Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 (1996).
Maeda, K. et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 221, 286–289 (1997).
Nakano, Y., Tobe, T., Choi-Miura, N.H., Mazda, T. & Tomita, M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. (Tokyo) 120, 803–812 (1996).
Reichenberger, E. et al. Genomic organization and full-length cDNA sequence of human collagen X. FEBS Lett. 311, 305–310 (1992).
Reid, K.B., Gagnon, J. & Frampton, J. Completion of the amino acid sequences of the A and B chains of subcomponent C1q of the first component of human complement. Biochem. J. 203, 559–569 (1982).
Kondo, N. & Kondo, J. Identification of novel blood proteins specific for mammalian hibernation. J. Biol. Chem. 267, 473–478 (1992).
Shapiro, L. & Scherer, P.E. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 8, 335–338 (1998).
Hotta, K. et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 20, 1595–1599 (2000).
Fruebis, J. et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 98, 2005–2010 (2001).
Meng, Y.G., Liang, J., Wong, W.L. & Chisholm, V. Green fluorescent protein as a second selectable marker for selection of high producing clones from transfected CHO cells. Gene 242, 201–207 (2000).
Harlow, E. & Lane, D. Antibodies: A Laboratory Manual. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1988).
Cherrington, A.D. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 48, 1198–1214 (1999).
Seoane, J. et al. Metabolic impact of adenovirus-mediated overexpression of the glucose-6- phosphatase catalytic subunit in hepatocytes. J. Biol. Chem. 272, 26972–26977 (1997).
Masoro, E.J. Caloric restriction and aging: an update. Exp. Gerontol. 35, 299–305 (2000).
Gupta, G. et al. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am. J. Physiol. Endocrinol. Metab. 278, E985–991. (2000).
Day, C. Thiazolidinediones: A new class of antidiabetic drugs. Diabet. Med. 16, 179–192 (1999).
Reginato, M.J. & Lazar, M.A. Mechanisms by which thiazolidinediones enhance insulin action. Trends Endocrinol. Metab. 10, 9–13 (1999).
Chao, L. et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest. 106, 1221–1228 (2000).
Kissebah, A.H. et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 97, 14478–14483 (2000).
Das, K., Lin, Y., Widen, E., Zhang, Y. & Scherer, P.E. Chromosomal localization, expression pattern and promoter analysis of the mouse gene encoding adipocyte-specific secretory protein Acrp30. Biochem. Biophys. Res. Comm. 280, 1120–1129 (2001).
Takahashi, M. et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int. J. Obes. Relat. Metab. Disord. 24, 861–868 (2000).
Lang, C.H., Bagby, G.J., Buday, A.Z. & Spitzer, J.J. The contribution of gluconeogenesis to glycogen repletion during glucose infusion in endotoxemia. Metabolism 36, 180–187 (1987).
Bligh, E. & Dyer, W.J. Rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Berry, M.N. & Friend, B.S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structure study. J. Cell. Biol. 43, 506–520 (1969).
Leffert, H.L., Koch, K.S., Moran, T. & Williams, M. Liver cells. Methods Enzymol. 58, 536–544 (1979).
Acknowledgements
We thank L. Rossetti, M. Charron and D. Stein for helpful discussions; D. Harrison and S. Klebanov for control and food-restricted serum samples; S. Nathenson and T. DiLorenzo for providing NOD mice for experiments; D. Neufeld for his help in isolating rat primary hepatocytes; and F. Mancia and A. Nemes for vector pFM1. This work was supported by the Training Program in Cellular & Molecular Biology & Genetics (T32-GM07491 to A.H.B.), the Hormones/Membrane Interactions Training Grant (NIH-T32 DK 07513-15 to T.C.), grants from the American Diabetes Association (to P.E.S.) and a NIH grant from the NIDDK (1R01-DK55758 to P.E.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berg, A., Combs, T., Du, X. et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7, 947–953 (2001). https://doi.org/10.1038/90992
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/90992
This article is cited by
-
Aging adipose tissue, insulin resistance, and type 2 diabetes
Biogerontology (2024)
-
Telmisartan and candesartan promote browning of white adipose tissue and reverse fatty liver changes in high fat diet fed male albino rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Novel targets for potential therapeutic use in Diabetes mellitus
Diabetology & Metabolic Syndrome (2023)
-
A comprehensive mechanistic model of adipocyte signaling with layers of confidence
npj Systems Biology and Applications (2023)
-
Daily phytate intake increases adiponectin levels among patients with diabetes type 2: a randomized crossover trial
Nutrition & Diabetes (2023)