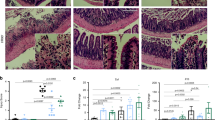
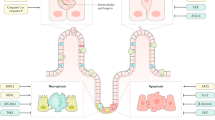
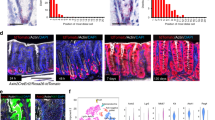
Abstract
Inflammation underlies many chronic and degenerative diseases, but it also mitigates infections, clears damaged cells and initiates tissue repair. Many of the mechanisms that link inflammation to damage repair and regeneration in mammals are conserved in lower organisms, indicating that it is an evolutionarily important process. Recent insights have shed light on the cellular and molecular processes through which conventional inflammatory cytokines and Wnt factors control mammalian tissue repair and regeneration. This is particularly important for regeneration in the gastrointestinal system, especially for intestine and liver tissues in which aberrant and deregulated repair results in severe pathologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011). This paper outlines how Paneth cells provide support for ISCs.
Durand, A. et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc. Natl Acad. Sci. USA 109, 8965–8970 (2012).
Yang, J. et al. β-catenin signaling in murine liver zonation and regeneration: a Wnt–Wnt situation! Hepatology 60, 964–976 (2014).
Wang, B., Zhao, L., Fish, M., Logan, C. Y. & Nusse, R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 524, 180–185 (2015). This paper describes a population of diploid pericentral hepatocytes that may act as adult liver stem cells.
Stanger, B. Z. Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol. 77, 179–200 (2015).
Sun, G. & Irvine, K. D. Control of growth during regeneration. Curr. Top. Dev. Biol. 108, 95–120 (2014).
Monga, S. P. Role and regulation of β-catenin signaling during physiological liver growth. Gene Expr. 16, 51–62 (2014).
Hu, M. et al. Wnt/β-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology 133, 1579–1591 (2007).
Farber, E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3′-methyl-4-dimethylaminoazobenzene. Cancer Res. 16, 142–148 (1956).
Popper, H., Kent, G. & Stein, R. Ductular cell reaction in the liver in hepatic injury. J. Mt. Sinai Hosp. 24, 551–556 (1957).
West, N. R., McCuaig, S., Franchini, F. & Powrie, F. Emerging cytokine networks in colorectal cancer. Nature Rev. Immunol. 15, 615–629 (2015).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Stramer, B. M., Mori, R. & Martin, P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Invest. Dermatol. 127, 1009–1017 (2007).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Panayidou, S. & Apidianakis, Y. Regenerative inflammation: lessons from Drosophila intestinal epithelium in health and disease. Pathogens 2, 209–231 (2013).
Shaukat, Z., Liu, D. & Gregory, S. Sterile inflammation in Drosophila. Mediators Inflamm. 2015, 369286 (2015).
Buchon, N., Silverman, N. & Cherry, S. Immunity in Drosophila melanogaster — from microbial recognition to whole-organism physiology. Nature Rev. Immunol. 14, 796–810 (2014).
Ayyaz, A. & Jasper, H. Intestinal inflammation and stem cell homeostasis in aging Drosophila melanogaster. Front. Cell Infect. Microbiol. 3, 98 (2013).
Clevers, H., Loh, K. M. & Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012 (2014).
Xu, N. et al. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 354, 31–43 (2011).
Cordero, J. B., Stefanatos, R. K., Scopelliti, A., Vidal, M. & Sansom, O. J. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. EMBO J. 31, 3901–3917 (2012).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Sommer, F. & Backhed, F. The gut microbiota — masters of host development and physiology. Nature Rev. Microbiol. 11, 227–238 (2013).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004). This is one of the first reports to describe the role of TLR4 signalling in control of mucosal homeostasis.
Neurath, M. F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 7, 6–19 (2014).
Claud, E. C. Neonatal necrotizing enterocolitis — inflammation and intestinal immaturity. Antiinflamm. Antiallergy Agents Med. Chem. 8, 248–259 (2009).
Lee, W. J. & Brey, P. T. How microbiomes influence metazoan development: insights from history and Drosophila modeling of gut–microbe interactions. Annu. Rev. Cell Dev. Biol. 29, 571–592 (2013).
Cornell, R. P., Liljequist, B. L. & Bartizal, K. F. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology 11, 916–922 (1990).
Seki, E. et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology 41, 443–450 (2005). This is one of the first accounts of the control of liver regeneration by TLR signalling.
Rayes, N. et al. Effect of pre- and probiotics on liver regeneration after resection: a randomised, double-blind pilot study. Benef. Microbes 3, 237–244 (2012).
Taub, R. Liver regeneration: from myth to mechanism. Nature Rev. Mol. Cell Biol. 5, 836–847 (2004).
Cressman, D. E. et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274, 1379–1383 (1996). This key paper describes the regenerative function of IL-6.
DeAngelis, R. A. et al. A complement-IL-4 regulatory circuit controls liver regeneration. J. Immunol. 188, 641–648 (2012).
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Elliott, E. I. & Sutterwala, F. S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 265, 35–52 (2015).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Lopetuso, L. R., Chowdhry, S. & Pizarro, T. T. Opposing functions of classic and novel IL-1 family members in gut health and disease. Front. Immunol. 4, 181 (2013).
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1–13 (2015).
Stevens, L. J. & Page-McCaw, A. A secreted MMP is required for re-epithelialization during wound healing. Mol. Biol. Cell 23, 1068–1079 (2012).
Chalaris, A. et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Exp. Med. 207, 1617–1624 (2010).
Scheller, J., Chalaris, A., Garbers, C. & Rose-John, S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 32, 380–387 (2011).
Yamada, Y., Kirillova, I., Peschon, J. J. & Fausto, N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl Acad. Sci. USA 94, 1441–1446 (1997).
Liu, Z. G., Hsu, H., Goeddel, D. V. & Karin, M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87, 565–576 (1996).
Beg, A. A. & Baltimore, D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274, 782–784 (1996).
Kamata, H. et al. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120, 649–661 (2005).
Marshall, K. M., He, S., Zhong, Z., Atkinson, C. & Tomlinson, S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J. Exp. Med. 211, 1793–1805 (2014).
Chen, L. W. et al. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nature Med. 9, 575–581 (2003). This is the first account of the crucial protective and regenerative function of TNF-induced intestinal NF-κB.
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Becker, C. et al. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004).
Bohm, F., Kohler, U. A., Speicher, T. & Werner, S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol. Med. 2, 294–305 (2010).
Akerman, P. et al. Antibodies to tumor necrosis factor-α inhibit liver regeneration after partial hepatectomy. Am. J. Physiol. 263, G579–G585 (1992).
Yamada, Y., Webber, E. M., Kirillova, I., Peschon, J. J. & Fausto, N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology 28, 959–970 (1998).
Nakagawa, H. et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343 (2014).
Ando, K. et al. Induction of Notch signaling by tumor necrosis factor in rheumatoid synovial fibroblasts. Oncogene 22, 7796–7803 (2003).
Hilliard, V. C., Frey, M. R., Dempsey, P. J., Peek, R. M. Jr & Polk, D. B. TNF-α converting enzyme-mediated ErbB4 transactivation by TNF promotes colonic epithelial cell survival. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G338–G346 (2011).
Anders, R. A., Subudhi, S. K., Wang, J., Pfeffer, K. & Fu, Y. X. Contribution of the lymphotoxin β receptor to liver regeneration. J. Immunol. 175, 1295–1300 (2005).
Garbers, C. et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 23, 85–97 (2012).
Kishimoto, T. IL-6: from its discovery to clinical applications. Int. Immunol. 22, 347–352 (2010).
Brandl, K. et al. MyD88 signaling in non-hematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc. Natl Acad. Sci. USA 107, 19967–19972 (2010).
Makki, N., Thiel, K. W. & Miller, F. J. Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int. J. Mol. Sci. 14, 20597–20613 (2013).
Taniguchi, K. et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature 519, 57–62 (2015). This key paper describes the role of gp130-induced, Hippo-independent YAP signalling in epithelial regeneration.
Nikoopour, E., Bellemore, S. M. & Singh, B. IL-22, cell regeneration and autoimmunity. Cytokine 74, 35–42 (2015).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature Med. 14, 282–289 (2008).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Zenewicz, L. A. et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659 (2007). This paper is an important account of the unique regenerative function of IL-22.
Radaeva, S., Sun, R., Pan, H. N., Hong, F. & Gao, B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39, 1332–1342 (2004).
Pappu, R., Rutz, S. & Ouyang, W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 33, 343–349 (2012).
Hymowitz, S. G. et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 20, 5332–5341 (2001).
Ely, L. K., Fischer, S. & Garcia, K. C. Structural basis of receptor sharing by interleukin 17 cytokines. Nature Immunol. 10, 1245–1251 (2009).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Song, X. et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nature Immunol. 12, 1151–1158 (2011).
Wang, K. et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity 41, 1052–1063 (2014).
Stepniak, E. et al. c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev. 20, 2306–2314 (2006).
Sakurai, T., Maeda, S., Chang, L. & Karin, M. Loss of hepatic NF-κB activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc. Natl Acad. Sci. USA 103, 10544–10551 (2006).
Chang, L. et al. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIPL turnover. Cell 124, 601–613 (2006).
Papa, S. et al. Gadd45β promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J. Clin. Invest. 118, 1911–1923 (2008).
Schwabe, R. F. & Brenner, D. A. Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G583–G589 (2006).
Hasselblatt, P., Rath, M., Komnenovic, V., Zatloukal, K. & Wagner, E. F. Hepatocyte survival in acute hepatitis is due to c-Jun/AP-1-dependent expression of inducible nitric oxide synthase. Proc. Natl Acad. Sci. USA 104, 17105–17110 (2007).
Seki, E., Brenner, D. A. & Karin, M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143, 307–320 (2012).
Jiang, H., Grenley, M. O., Bravo, M. J., Blumhagen, R. Z. & Edgar, B. A. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 (2011).
Frey, M. R., Golovin, A. & Polk, D. B. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J. Biol. Chem. 279, 44513–44521 (2004).
Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nature Immunol. 12, 715–723 (2011).
Maeda, S. et al. IKKβ is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFα. Immunity 19, 725–737 (2003).
Maeda, S., Kamata, H., Luo, J. L., Leffert, H. & Karin, M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121, 977–990 (2005).
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004). This is the first account of the crucial tumour-promoting function of NF-κB signalling in intestinal epithelial cells and macrophages.
Egan, L. J. et al. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc. Natl Acad. Sci. USA 101, 2452–2457 (2004).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Eckmann, L. et al. Opposing functions of IKKβ during acute and chronic intestinal inflammation. Proc. Natl Acad. Sci. USA 105, 15058–15063 (2008).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Jiang, H. & Edgar, B. A. Intestinal stem cell function in Drosophila and mice. Curr. Opin. Genet. Dev. 22, 354–360 (2012).
Ernst, M., Thiem, S., Nguyen, P. M., Eissmann, M. & Putoczki, T. L. Epithelial gp130/Stat3 functions: an intestinal signaling node in health and disease. Semin. Immunol. 26, 29–37 (2014).
Kolls, J. K., McCray, P. B. Jr & Chan, Y. R. Cytokine-mediated regulation of antimicrobial proteins. Nature Rev. Immunol. 8, 829–835 (2008).
Wittkopf, N. et al. Activation of intestinal epithelial Stat3 orchestrates tissue defense during gastrointestinal infection. PLoS ONE 10, e0118401 (2015).
Grivennikov, S. I. & Karin, M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 21, 11–19 (2010).
Bollrath, J. et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15, 91–102 (2009).
Anderson, C. A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nature Genet. 43, 246–252 (2011).
Moh, A. et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab. Invest. 87, 1018–1028 (2007).
He, G. et al. Hepatocyte IKKβ/NF-κB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 17, 286–297 (2010).
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nature Rev. Drug Discov. 13, 63–79 (2014).
Baddour, L. M., Cha, Y. M. & Wilson, W. R. Clinical practice. Infections of cardiovascular implantable electronic devices. N. Engl. J. Med. 367, 842–849 (2012).
Zhang, J. et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nature Cell Biol. 11, 1444–1450 (2009).
Tschaharganeh, D. F. et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 144, 1530–1542 (2013).
Yu, F. X. & Guan, K. L. The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 (2013).
Karpowicz, P., Perez, J. & Perrimon, N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137, 4135–4145 (2010).
Ren, F. et al. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc. Natl Acad. Sci. USA 107, 21064–21069 (2010).
Shaw, R. L. et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137, 4147–4158 (2010).
Staley, B. K. & Irvine, K. D. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr. Biol. 20, 1580–1587 (2010).
Zhou, D. et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl Acad. Sci. USA 108, e1312–e1320 (2011).
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007).
Cai, J. et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 24, 2383–2388 (2010).
Fre, S. et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435, 964–968 (2005).
Stanger, B. Z., Datar, R., Murtaugh, L. C. & Melton, D. A. Direct regulation of intestinal fate by Notch. Proc. Natl Acad. Sci. USA 102, 12443–12448 (2005).
van Es, J. H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005). References 115 to 117 describe the crucial role of Notch signalling in the control of stem-cell fate in the mammalian gut.
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006).
Cordero, J. B. et al. c-Src drives intestinal regeneration and transformation. EMBO J. 33, 1474–1491 (2014).
Korinek, V. et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet. 19, 379–383 (1998).
Liu, S. et al. Lgr4 gene deficiency increases susceptibility and severity of dextran sodium sulfate-induced inflammatory bowel disease in mice. J. Biol. Chem. 288, 8794–8803 (2013).
Ashton, G. H. et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell 19, 259–269 (2010).
Miyoshi, H., Ajima, R., Luo, C. T., Yamaguchi, T. P. & Stappenbeck, T. S. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113 (2012). This paper is an important account of the key parts played by Wnt and TGFβ in control of intestinal regeneration.
Clevers, H. C. & Bevins, C. L. Paneth cells: maestros of the small intestinal crypts. Annu. Rev. Physiol. 75, 289–311 (2013).
Potten, C. S. Extreme sensitivity of some intestinal crypt cells to X and γ irradiation. Nature 269, 518–521 (1977).
Sangiorgi, E. & Capecchi, M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nature Genet. 40, 915–920 (2008).
Munoz, J. et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. EMBO J. 31, 3079–3091 (2012).
Roche, K. C. et al. SOX9 maintains reserve stem cells and preserves radio-resistance in mouse small intestine. Gastroenterology 149, 1553–1563 (2015).
Buczacki, S. J. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Choi, T. Y., Ninov, N., Stainier, D. Y. & Shin, D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 146, 776–788 (2014).
Grompe, M. Liver stem cells, where art thou? Cell Stem Cell 15, 257–258 (2014).
Font-Burgada, J. et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766–779 (2015). This paper shows that periportal hepatocytes rather than oval cells are responsible for liver regeneration after injury, but do not give rise to cancer.
Apte, U. et al. Wnt/β-catenin signaling mediates oval cell response in rodents. Hepatology 47, 288–295 (2008).
Itoh, T., Kamiya, Y., Okabe, M., Tanaka, M. & Miyajima, A. Inducible expression of Wnt genes during adult hepatic stem/progenitor cell response. FEBS Lett. 583, 777–781 (2009).
Yang, W. et al. Wnt/β-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 68, 4287–4295 (2008).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Boulter, L. et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nature Med. 18, 572–579 (2012).
Park, H. W. et al. Alternative Wnt signaling activates YAP/TAZ. Cell 162, 780–794 (2015).
Chung, A. S. et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nature Med. 19, 1114–1123 (2013).
Acknowledgements
The authors thank H. Gehart for producing the figures. Owing to space limitations, primary findings have been cited through reviews. Work by M.K., who is an American Cancer Society Research Professor and holder of the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases, is supported by the US National Institutes of Health, the Alliance for Lupus Research, the Lymphoma and Leukemia Society and the Superfund Basic Research Program. H.C. is supported by Stand Up to Cancer, the European Research Council, Alpe d'HuZes/KWF and the Netherlands Research Council NWO.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Karin, M., Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315 (2016). https://doi.org/10.1038/nature17039
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17039
This article is cited by
-
Resveratrol-βcd inhibited premature ovarian insufficiency progression by regulating granulosa cell autophagy
Journal of Ovarian Research (2024)
-
Effects of dietary small peptides on growth, serum biochemistry, antioxidant status, and immune responses in juvenile snakehead (Channa argus)
Aquaculture International (2024)
-
Physiological and pathological consequences of exosomes at the blood–brain-barrier interface
Cell Communication and Signaling (2023)
-
Human adipose tissue-derived small extracellular vesicles promote soft tissue repair through modulating M1-to-M2 polarization of macrophages
Stem Cell Research & Therapy (2023)
-
Advances in radiotherapy and immunity in hepatocellular carcinoma
Journal of Translational Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.