Abstract
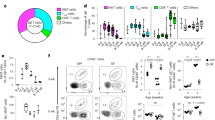
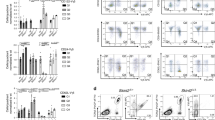
NKp46+CD3− natural killer lymphocytes isolated from blood, lymphoid organs, lung, liver and uterus can produce granule-dependent cytotoxicity and interferon-γ. Here we identify in dermis, gut lamina propria and cryptopatches distinct populations of NKp46+CD3− cells with a diminished capacity to degranulate and produce interferon-γ. In the gut, expression of the transcription factor RORγt, which is involved in the development of lymphoid tissue–inducer cells, defined a previously unknown subset of NKp46+CD3− lymphocytes. Unlike RORγt− lamina propria and dermis natural killer cells, gut RORγt+NKp46+ cells produced interleukin 22. Our data show that lymphoid tissue–inducer cells and natural killer cells shared unanticipated similarities and emphasize the heterogeneity of NKp46+CD3− cells in innate immunity, lymphoid organization and local tissue repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Kupper, T.S. & Fuhlbrigge, R.C. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 4, 211–222 (2004).
Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 25, 381–418 (2007).
Tlaskalova-Hogenova, H. et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 93, 97–108 (2004).
Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3, 331–341 (2003).
Jameson, J. & Havran, W.L. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol. Rev. 215, 114–122 (2007).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Hayakawa, Y., Huntington, N.D., Nutt, S.L. & Smyth, M.J. Functional subsets of mouse natural killer cells. Immunol. Rev. 214, 47–55 (2006).
Gregoire, C. et al. The trafficking of natural killer cells. Immunol. Rev. 220, 169–182 (2007).
Eiras, P. et al. Intestinal intraepithelial lymphocytes contain a CD3−CD7+ subset expressing natural killer markers and a singular pattern of adhesion molecules. Scand. J. Immunol. 52, 1–6 (2000).
Cohavy, O., Zhou, J., Ware, C.F. & Targan, S.R. LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J. Immunol. 174, 646–653 (2005).
McDermott, J.R., Humphreys, N.E., Forman, S.P., Donaldson, D.D. & Grencis, R.K. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J. Immunol. 175, 3207–3213 (2005).
Harrington, L., Srikanth, C.V., Antony, R., Shi, H.N. & Cherayil, B.J. A role for natural killer cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol. Med. Microbiol. 76, 3045–3053 (2007).
Ebert, L.M., Meuter, S. & Moser, B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J. Immunol. 176, 4331–4336 (2006).
Ottaviani, C. et al. CD56brightCD16− NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur. J. Immunol. 36, 118–128 (2005).
Buentke, E. et al. Natural killer and dendritic cell contact in lesional atopic dermatitis skin–Malassezia-influenced cell interaction. J. Invest. Dermatol. 119, 850–857 (2002).
Parolini, S. et al. The role of chemerin in the co-localization of NK and dendritic cell subsets into inflamed tissues. Blood 109, 3625–3632 (2007).
Walzer, T. et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA 104, 3384–3389 (2007).
Stewart, C.A. et al. Germ-line and rearranged Tcrd transcription distinguish bona fide NK cells and NK-like γδ T cells. Eur. J. Immunol. 37, 1442–1452 (2007).
Kim, S. et al. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 3, 523–528 (2002).
Jamieson, A.M., Isnard, P., Dorfman, J.R., Coles, M.C. & Raulet, D.H. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J. Immunol. 172, 864–870 (2004).
Hayakawa, Y. & Smyth, M.J. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176, 1517–1524 (2006).
Eberl, G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat. Rev. Immunol. 5, 413–420 (2005).
Mebius, R.E., Rennert, P. & Weissman, I.L. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Eberl, G. & Littman, D.R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Dong, C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8, 337–348 (2008).
Zhou, L. et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453, 236–240 (2008).
Aujla, S.J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281 (2008).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Ouyang, W., Kolls, J.K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
O'Leary, J.G., Goodarzi, M., Drayton, D.L. & von Andrian, U.H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 (2006).
Vosshenrich, C.A. et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 7, 1217–1224 (2006).
Caligiuri, M.A. Human natural killer cells. Blood 112, 461–469 (2008).
Sanos, S.L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nat. Immunol. advance online publication, doi:10.1038/ni1684 (23 November 2008).
Meresse, B. et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 203, 1343–1345 (2006).
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999).
Boos, M.D., Yokota, Y., Eberl, G. & Kee, B.L. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 204, 1119–1130 (2007).
Cupedo, T. et al. Human fetal lymphoid tissue–inducer cells are interleukin 17–producing precursors to RORC+ CD127+ natural killer–like cells. Nat. Immunol. advance online publication, doi:10.1038/ni1668 (23 November 2008).
Pessino, A. et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 188, 953–960 (1998).
Gazit, R. et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7, 517–523 (2006).
Mandelboim, O. et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409, 1055–1060 (2001).
Nolte-'t Hoen, E. et al. Increased surveillance of cells in mitosis by human NK cells suggests a novel strategy for limiting tumor growth and viral replication. Blood 109, 670–673 (2006).
Scandella, E. et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat. Immunol. 9, 667–675 (2008).
Kim, M.Y. et al. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology 124, 166–174 (2008).
Wolk, K. & Sabat, R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 17, 367–380 (2006).
Zenewicz, L.A. et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659 (2007).
Bangert, C., Friedl, J., Stary, G., Stingl, G. & Kopp, T. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Invest. Dermatol. 121, 1409–1418 (2003).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Spaggiari, G.M. et al. Soluble HLA class I induces NK cell apoptosis upon the engagement of killer-activating HLA class I receptors through FasL-Fas interaction. Blood 100, 4098–4107 (2002).
Chaix, J. et al. Cutting edge: Priming of NK cells by IL-18. J. Immunol. 181, 1627–1631 (2008).
Acknowledgements
We thank the mouse functional genomics platform of Marseille-Nice Genopole for immunohistology; P. Grenot, M. Barad and A. Zouine from the Centre d'Immunologie de Marseille-Luminy flow cytometry core facility and M. Fallet from the Centre d'Immunologie de Marseille-Luminy microscopy facility for assistance; M. Betizeau for contributions to the initial phases of this work; M. Baratin, T. Walzer and S. Ugolini for discussions; and M. Ito (Central Institute for Experimental Animals) for Rag2−/−Il2rg−/− mice. Anti–human NKVSF1 was provided by A. Poggi (National Cancer Institute, Genoa, Italy), and anti-IL-22 (MH22B2) was provided by J.C. Renauld (Ludwig Institute for Cancer Research). E.V. and E.T. are joint senior authors. Supported by Ligue Nationale contre le Cancer ('Equipe labellisée La Ligue'), Agence Nationale de la Recherche, Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Ministère de l'Enseignement Supérieur et de la Recherche, Fondation pour la Recherche Medicale (C.L.), Region Provence Alpes Côte d′Azur–Institut National de la Santé et de la Recherche Médicale (A.R.), the Crohn's and Colitis Foundation of America (I.I.I.) and the Institut Universitaire de France (E.V.).
Author information
Authors and Affiliations
Contributions
E.T. and E.V. designed the experiments and wrote the paper; E.T., C.L., A.R., I.I.I. and C.C. did and analyzed the experiments; Li.C. contributed to tissue section preparation and immunofluorescence staining; J.H. provided human samples; E.A., J.B. and D.C. did microarray hybridization and preliminary analysis; La.C. and M.D. contributed to microarray data analysis; and D.R.L. provided reagents crucial for the experiments and contributed to the design of the experiments and the interpretation of the data.
Corresponding authors
Ethics declarations
Competing interests
EV is a cofounder and shareholder of Innate-Pharma.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–2, Supplementary Tables 1–2 and Supplementary Methods (PDF 3858 kb)
Rights and permissions
About this article
Cite this article
Luci, C., Reynders, A., Ivanov, I. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat Immunol 10, 75–82 (2009). https://doi.org/10.1038/ni.1681
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1681
This article is cited by
-
The emerging family of RORγt+ antigen-presenting cells
Nature Reviews Immunology (2024)
-
Versatile roles of innate lymphoid cells at the mucosal barrier: from homeostasis to pathological inflammation
Experimental & Molecular Medicine (2023)
-
The unique role of innate lymphoid cells in cancer and the hepatic microenvironment
Cellular & Molecular Immunology (2022)
-
Innate lymphoid cells and cancer
Nature Immunology (2022)
-
ZBTB46 defines and regulates ILC3s that protect the intestine
Nature (2022)