Abstract
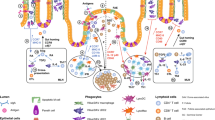
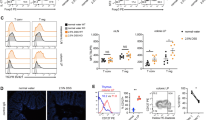
The control of damaging inflammation by the mucosal immune system in response to commensal and harmful ingested bacteria is unknown. Here we show epithelial cells conditioned mucosal dendritic cells through the constitutive release of thymic stromal lymphopoietin and other mediators, resulting in the induction of 'noninflammatory' dendritic cells. Epithelial cell–conditioned dendritic cells released interleukins 10 and 6 but not interleukin 12, and they promoted the polarization of T cells toward a 'classical' noninflammatory T helper type 2 response, even after exposure to a T helper type 1–inducing pathogen. This control of immune responses seemed to be lost in patients with Crohn disease. Thus, the intimate interplay between intestinal epithelial cells and dendritic cells may help to maintain gut immune homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
20 November 2014
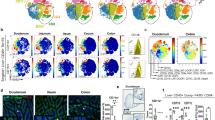
In the version of this article initially published, the plots at top and middle left in Figure 5a were incorrect. The correct plots are now presented. The error has been corrected in the HTML and PDF versions of the article.
References
Nagler-Anderson, C. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 1, 59–67 (2001).
Neutra, M.R., Mantis, N.J. & Kraehenbuhl, J.P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2, 1004–1009 (2001).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2, 361–367 (2001).
Niess, J.H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Rescigno, M. Dendritic cells and the complexity of microbial infection. Trends Microbiol. 10, 425–461 (2002).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Bilsborough, J. & Viney, J.L. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology 127, 300–309 (2004).
Alpan, O., Rudomen, G. & Matzinger, P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J. Immunol. 166, 4843–4852 (2001).
Akbari, O., DeKruyff, R.H. & Umetsu, D.T. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2, 725–731 (2001).
Iwasaki, A. & Kelsall, B.L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1394 (2000).
Iwasaki, A. & Kelsall, B.L. Unique functions of CD11b+, CD8α+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166, 4884–4890 (2001).
Sato, A. et al. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J. Immunol. 171, 3684–3690 (2003).
Zhang, M. et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 5, 1124–1133 (2004).
McWilliam, A.S. et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J. Exp. Med. 184, 2429–2432 (1996).
Trinchieri, G., Pflanz, S. & Kastelein, R.A. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19, 641–644 (2003).
Reche, P.A. et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167, 336–343 (2001).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3, 673–680 (2002).
Soumelis, V. & Liu, Y.J. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin. Immunopathol. 25, 325–333 (2004).
Gilliet, M. et al. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J. Exp. Med. 197, 1059–1063 (2003).
Watanabe, N. et al. Human thymic stromal lymphopoietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 5, 426–434 (2004).
Rimoldi, M., Chieppa, M., Vulcano, M., Allavena, P. & Rescigno, M. Intestinal epithelial cells control DC function. Ann. NY Acad. Sci. 1029, 66–74 (2004).
Bouma, G. & Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3, 521–533 (2003).
Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3, 331–341 (2003).
Anjuere, F. et al. Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses. J. Immunol. 170, 1586–1592 (2003).
Macpherson, A.J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000).
Macpherson, A.J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Jang, M.H. et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA 101, 6110–6115 (2004).
Mastroeni, P. & Menager, N. Development of acquired immunity to Salmonella. J. Med. Microbiol. 52, 453–459 (2003).
Hess, J., Ladel, C., Miko, D. & Kaufmann, S.H. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-αβ cells and IFN-γ in bacterial clearance independent of intracellular location. J. Immunol. 156, 3321–3326 (1996).
George, A. Generation of gamma interferon responses in murine Peyer's patches following oral immunization. Infect. Immun. 64, 4606–4611 (1996).
Liesenfeld, O., Kosek, J.C. & Suzuki, Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect. Immun. 65, 4682–4689 (1997).
Vossenkamper, A. et al. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur. J. Immunol. 34, 3197–3207 (2004).
Sierro, F. et al. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA 98, 13722–13727 (2001).
Vanbervliet, B. et al. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: possible role at inflamed epithelial surfaces. Eur. J. Immunol. 32, 231–242 (2002).
Dieu-Nosjean, M.C. et al. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192, 705–718 (2000).
Eckmann, L. & Kagnoff, M.F. Cytokines in host defense against Salmonella. Microbes Infect. 3, 1191–1200 (2001).
Neish, A.S. et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 289, 1560–1563 (2000).
Kelly, D. et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 5, 104–112 (2004).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 (1994).
Whither RNAi? Nat. Cell Biol. 5, 489–490 (2003).
Acknowledgements
We thank S. Chiocca and J.P. Kraehenbühl for critical reading of the manuscript; P. Larghi for continuous experimental help; E. Colli and F. Dalla Valle for technical assistance; and E. Torchiana from Miltenyi Biotech for technical support. Supported by Fondazione Italiana per la Ricerca sul Cancro (M.C.) and the Crohn's and Colitis Foundation of America and Associazione Italiana per la Ricerca sul Cancro, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
MoDCs conditioned with Caco-2 supernatant show similar response to bacteria in terms of changes in morphology and surface markers. (PDF 1527 kb)
Supplementary Fig. 2
Naive T cells stimulated with conditioned MoDCs proliferate similarly, regardless of treatment. (PDF 188 kb)
Supplementary Fig. 3
Naive T cells stimulated with conditioned MoDCs proliferate similarly, regardless of treatment. (PDF 187 kb)
Rights and permissions
About this article
Cite this article
Rimoldi, M., Chieppa, M., Salucci, V. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol 6, 507–514 (2005). https://doi.org/10.1038/ni1192
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1192
This article is cited by
-
Immunomodulatory effect of probiotic exopolysaccharides in a porcine in vitro co-culture model mimicking the intestinal environment on ETEC infection
Veterinary Research Communications (2023)
-
The role of retinoic acid in the production of immunoglobulin A
Mucosal Immunology (2022)
-
Dendritic cell functions in the inductive and effector sites of intestinal immunity
Mucosal Immunology (2022)
-
Epithelial Toll-like receptors and their role in gut homeostasis and disease
Nature Reviews Gastroenterology & Hepatology (2020)
-
Inflammatory Responses of Porcine MoDC and Intestinal Epithelial Cells in a Direct-Contact Co-culture System Following a Bacterial Challenge
Inflammation (2020)