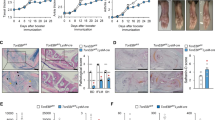
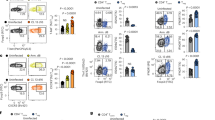
Abstract
Immune sensing of a microbe occurs via multiple receptors. How signals from different receptors are coordinated to yield a specific immune response is poorly understood. We show that two pathogen recognition receptors, Toll-like receptor 2 (TLR2) and dectin-1, recognizing the same microbial stimulus, stimulate distinct innate and adaptive responses. TLR2 signaling induced splenic dendritic cells (DCs) to express the retinoic acid metabolizing enzyme retinaldehyde dehydrogenase type 2 and interleukin-10 (IL-10) and to metabolize vitamin A and stimulate Foxp3+ T regulatory cells (Treg cells). Retinoic acid acted on DCs to induce suppressor of cytokine signaling-3 expression, which suppressed activation of p38 mitogen-activated protein kinase and proinflammatory cytokines. Consistent with this finding, TLR2 signaling induced Treg cells and suppressed IL-23 and T helper type 17 (TH17) and TH1-mediated autoimmune responses in vivo. In contrast, dectin-1 signaling mostly induced IL-23 and proinflammatory cytokines and augmented TH17 and TH1-mediated autoimmune responses in vivo. These data define a new mechanism for the systemic induction of retinoic acid and immune suppression against autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shortman, K. & Liu, Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161 (2002).
Pulendran, B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 199, 227–250 (2004).
Wu, L. & Liu, Y.J. Development of dendritic-cell lineages. Immunity 26, 741–750 (2007).
Palucka, A.K., Ueno, H., Fay, J.W. & Banchereau, J. Taming cancer by inducing immunity via dendritic cells. Immunol. Rev. 220, 129–150 (2007).
Steinman, R.M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Pasare, C. & Medzhitov, R. Toll-like receptors and acquired immunity. Semin. Immunol. 16, 23–26 (2004).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Van Kooyk, Y. & Geijtenbeek, T.B. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3, 697–709 (2003).
Brown, G.D. & Gordon, S. Immune recognition. A new receptor for β-glucans. Nature 413, 36–37 (2001).
Brown, G.D. et al. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196, 407–412 (2002).
Underhill, D.M. et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 (1999).
Gantner, B.N., Simmons, R.M., Canavera, S.J, Akira, S. & Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003).
Underhill, D.M. Collaboration between the innate immune receptors dectin-1, TLRs and Nods. Immunol. Rev. 219, 75–87 (2007).
Brown, G.D. et al. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 (2003).
Rogers, N.C. et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005).
Slack, E.C. et al. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur. J. Immunol. 37, 1600–1612 (2007).
Dillon, S. et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116, 916–928 (2006).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17–producing T cells. Immunity 24, 179–189 (2006).
Zhou, L. et al. TGF-β–induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453, 236–240 (2008).
Dong, C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8, 337–348 (2008).
Yoshitomi, H. et al. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 (2005).
LeibundGut-Landmann, S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8, 630–638 (2007).
Veldhoen, M., Hocking, R.J., Flavell, R.A. & Stockinger, B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 7, 1151–1156 (2006).
Sun, C.M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Denning, T.L., Wang, Y.C., Patel, S.R., Williams, I.R. & Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17–producing T cell responses. Nat. Immunol. 8, 1086–1094 (2007).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid–dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Benson, M.J., Pino-Lagos, K., Rosemblatt, M. & Noelle, R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204, 1765–1774 (2007).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Cao, W. et al. Toll-like receptor–mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI3K-mTOR-p70S6K pathway. Nat. Immunol. 9, 1157–1164 (2008).
Singh, M., Chakrapani, A. & O' Hagan, D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev. Vaccines 6, 797–808 (2007).
Mora, J.R. et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006).
Johansson-Lindbom, B. & Agace, W.W. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 215, 226–242 (2007).
Zapata-Gonzalez, F. et al. 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator–activated receptor γ blocks some of the 9cRA activities, and precludes them to mature phenotype development. J. Immunol. 178, 6130–6139 (2007).
Saurer, L., McCullough, K.C. & Summerfield, A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 179, 3504–3514 (2007).
Geissmann, F. et al. Retinoids regulate survival and antigen presentation by immature dendritic cells. J. Exp. Med. 198, 623–634 (2003).
Tao, Y., Yang, Y. & Wang, W. Effect of all-trans-retinoic acid on the differentiation, maturation and functions of dendritic cells derived from cord blood monocytes. FEMS Immunol. Med. Microbiol. 47, 444–450 (2006).
Bastien, J. & Rochette-Egly, C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 328, 1–16 (2004).
de The, H., Vivanco-Ruiz, M.M., Tiollais, P., Stunnenberg, H. & Dejean, A. Identification of a retinoic acid responsive element in the retinoic acid receptor β gene. Nature 343, 177–180 (1990).
Duester, G., Shean, M.L., McBride, M.S. & Stewart, M.J. Retinoic acid response element in the human alcohol dehydrogenase gene ADH3: implications for regulation of retinoic acid synthesis. Mol. Cell. Biol. 11, 1638–1646 (1991).
Leroy, P., Nakshatri, H. & Chambon, P. Mouse retinoic acid receptor α 2 isoform is transcribed from a promoter that contains a retinoic acid response element. Proc. Natl. Acad. Sci. USA 88, 10138–10142 (1991).
Szatmari, I. et al. PPARγ controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 203, 2351–2362 (2006).
Manicassamy, S. & Pulendran, B. Retinoic acid–dependent regulation of immune responses by dendritic cells and macrophages. Semin. Immunol. 21, 22–27 (2009).
Kubo, M., Hanada, T. & Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 4, 1169–1176 (2003).
Shouda, T. et al. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J. Clin. Invest. 108, 1781–1788 (2001).
Dalpke, A.H., Opper, S., Zimmermann, S. & Heeg, K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J. Immunol. 166, 7082–7089 (2001).
Agrawal, S. et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct TH responses via differential modulation of extracellular signal-regulated kinase–mitogen-activated protein kinase and c-Fos. J. Immunol. 171, 4984–4989 (2003).
Dillon, S. et al. A Toll-like receptor 2 ligand stimulates TH2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 172, 4733–4743 (2004).
Gerosa, F. et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 205, 1447–1461 (2008).
Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat. Immunol. 6, 769–776 (2005).
Acknowledgements
We thank S. Aguilar Mertens and L. Bonner for assistance with cell sorting; D. Levesque for assistance with animal husbandry; S. Akira (Research Institute for Microbial Diseases, Osaka University) for supplying Tlr2−/− mice; G. Brown (University of Cape Town) for dectin-1–deficient mice; and G. Landreth (Case Western Reserve University) for Erk1-deficient mice. This work was supported by funding from the US National Institutes of Health (grants R01 DK057665, R01 AI048638, U19 AI057266, U54 AI057157, N01 AI50019 and N01 AI50025) and from the Bill & Melinda Gates Foundation to B.P.
Author information
Authors and Affiliations
Contributions
S.M. performed the experiments in the study, prepared the figures and helped write the paper. R.R. assisted S.M. with some of the experiments. J.D. performed some of the initial experiments. H.O. performed the immunohistology analysis. T.L.D. provided discussion and advice on some parts of the study. S.P.K. encapsulated disulphiram in microparticles. K.M.R. and B.D.E. assisted with the EAE experiments. B.P. conceived and supervised the study and, with S.M., wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–12 and Supplementary Methods (PDF 1100 kb)
Rights and permissions
About this article
Cite this article
Manicassamy, S., Ravindran, R., Deng, J. et al. Toll-like receptor 2–dependent induction of vitamin A–metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med 15, 401–409 (2009). https://doi.org/10.1038/nm.1925
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1925
This article is cited by
-
The immunomodulatory role of all-trans retinoic acid in tumor microenvironment
Clinical and Experimental Medicine (2022)
-
All-trans retinoic acid inhibits lipopolysaccharide-induced inflammatory responses in bovine adipocytes via TGFβ1/Smad3 signaling pathway
BMC Veterinary Research (2019)
-
Age-associated antigen-presenting cell alterations promote dry-eye inducing Th1 cells
Mucosal Immunology (2019)
-
Retinoids as an Immunity-modulator in Dermatology Disorders
Archivum Immunologiae et Therapiae Experimentalis (2019)
-
Mechanisms of T cell organotropism
Cellular and Molecular Life Sciences (2016)