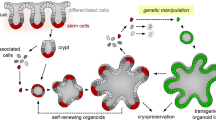
Abstract
Gene modification in untransformed human intestinal cells is an attractive approach for studying gene function in intestinal diseases. However, because of the lack of practical tools, such studies have largely depended upon surrogates, such as gene-engineered mice or immortalized human cell lines. By taking advantage of the recently developed intestinal organoid culture method, we developed a methodology for modulating genes of interest in untransformed human colonic organoids via electroporation of gene vectors. Here we describe a detailed protocol for the generation of intestinal organoids by culture with essential growth factors in a basement membrane matrix. We also describe how to stably integrate genes via the piggyBac transposon, as well as precise genome editing using the CRISPR-Cas9 system. Beginning with crypt isolation from a human colon sample, genetically modified organoids can be obtained in 3 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
05 December 2018
The version of this paper originally published shows incorrect units for two plasmid concentrations. In the "Reagent Setup" section, the instructions for sgRNA-Cas9 plasmid should read "Adjust the concentration of each plasmid to 1 μg μl–1,” rather than "to 1 μg ml–1.” Similarly, all concentrations in the tables in Steps 49A, 49C, and 49D should be in μg μl–1 instead of μg ml–1. Please note that these units have not been corrected in the PDF and HTML versions of the protocol available online.
References
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Koo, B.K. et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83 (2012).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 (2013).
Matano, M. et al. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Wang, F. et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145, 383–395 (2013).
Melkonyan, H., Sorg, C. & Klempt, M. Electroporation efficiency in mammalian cells is increased by dimethyl sulfoxide (DMSO). Nucleic Acids Res. 24, 4356–4357 (1996).
Ding, S. et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122, 473–483 (2005).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Schwank, G., Andersson-Rolf, A., Koo, B.K., Sasaki, N. & Clevers, H. Generation of BAC transgenic epithelial organoids. PLoS ONE 8, e76871 (2013).
Acknowledgements
This work was supported by grants from a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct), by a Grant-in-Aid for Scientific Research on Innovative Areas 'Stem Cell Aging and Disease' and by Grants-in-Aid for Scientific Research, Ministry of Education, Culture, Sports, Science and Technology of Japan. L-Wnt3A cells and the R-spondin1–producing cell line were kindly gifted by H. Clevers (Hubrecht Institute) and C. Kuo (Stanford University), respectively.
Author information
Authors and Affiliations
Contributions
M.F., M.M. and K.N. performed the experiments. T.S. conceived and designed the project. M.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.S. is an inventor on patents involving the organoid culture system (WO/2010/090513 and WO/2012/168930).
Integrated supplementary information
Supplementary Figure 1 Reduced growth efficiency by continuous treatment with CHIR99021.
Images of organoids treated with CHIR99021 for 7 days (left) and without CHIR99021 (with Wnt3A and R-Spondin1)(right) after single cell dissociation. Scale bars, 500 µm.
Supplementary Figure 2 Diagram of the comparisons for optimization of the electroporation method.
The conditions with red characters are superior to the others, and the protocol was optimized by combination of these conditions.
Supplementary Figure 3 Effect of filtration of the dissociated organoids.
Images of dissociated organoids before (left) and after (right) passing through a filter with 20-µm pores. Scale bars, 200 µm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 724 kb)
Rights and permissions
About this article
Cite this article
Fujii, M., Matano, M., Nanki, K. et al. Efficient genetic engineering of human intestinal organoids using electroporation. Nat Protoc 10, 1474–1485 (2015). https://doi.org/10.1038/nprot.2015.088
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.088
This article is cited by
-
Paneth-like cells produced from OLFM4+ stem cells support OLFM4+ stem cell growth in advanced colorectal cancer
Communications Biology (2024)
-
Chemically-defined and scalable culture system for intestinal stem cells derived from human intestinal organoids
Nature Communications (2024)
-
From cells to organs: progress and potential in cartilaginous organoids research
Journal of Translational Medicine (2023)
-
Bioengineering human intestinal mucosal grafts using patient-derived organoids, fibroblasts and scaffolds
Nature Protocols (2023)
-
Generating human blastoids modeling blastocyst-stage embryos and implantation
Nature Protocols (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.