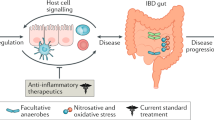
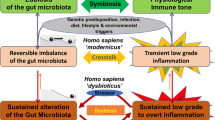
Abstract
Mucosal surfaces of the gut are colonized by large numbers of heterogeneous bacteria that contribute to intestinal health and disease. In genetically susceptible individuals, a 'pathogenic community' may arise, whereby abnormal gut flora contributes to alterations in the mucosa and local immune system leading to gastrointestinal disease. These diseases include enteric infections, such as Clostridium difficile infection, small intestinal bacterial overgrowth, functional gastrointestinal disorders (including IBS), IBD and colorectal cancer. Prebiotics, probiotics and synbiotics (a combination of prebiotics and probiotics) have the capacity to reverse pathologic changes in gut flora and local immunity. Intestinal health and disease need to be thoroughly characterized to understand the interplay between the indigenous microbiota, the immune system and genetic host factors. This Review provides a broad overview of the importance of the intestinal microbiota in chronic disorders of the gut.
Key Points
-
The intestinal microbiota consists of heterogeneous and largely nonculturable bacteria that orchestrate homeostasis by communicating with the epithelium and innate and adaptive immune mechanisms of the gut
-
Host immunity is directly involved in the control of the intestinal microbiota and susceptibility to chronic intestinal disease
-
Enteric infection by a high-grade bacterial pathogen can alter susceptibility to IBS by producing chronic intestinal inflammation and a series of events that leads to altered bowel function
-
Certain bacteria in the lumen of the gut can cross the intestinal mucosa, causing systemic infection in a process called 'translocation'
-
In IBD, abnormal gut flora (which may include pathogenic species) and the presence of bacterial antigens with biologic properties produce a 'pathogenic community', leading to intestinal inflammation
-
Modulation of the intestinal microbiota may be possible through diet or by administration of prebiotics, probiotics, synbiotics, or by fecal transplantation of donor stool from a healthy individual
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Zoetendal, E. G., Akkermans, A. D. & De Vos, W. M. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64, 3854–3859 (1998).
Mariat, D. et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 9, 123 (2009).
Dethlefsen, L., Huse, S., Sogin, M. L. & Relman, D. A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6, e280 (2008).
Jernberg, C., Lofmark, S., Edlund, C. & Jansson, J. K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156, 3216–3223 (2010).
Nikkila, J. & de Vos, W. M. Advanced approaches to characterize the human intestinal microbiota by computational meta-analysis. J. Clin. Gastroenterol. 44 (Suppl. 1), S2–S5 (2010).
Kinross, J. M., Darzi, A. W. & Nicholson, J. K. Gut microbiome-host interactions in health and disease. Genome Med. 3, 14 (2011).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Lee, J. et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat. Cell Biol. 8, 1327–1336 (2006).
Bauer, H., Horowitz, R. E., Levenson, S. M. & Popper, H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am. J. Pathol. 42, 471–483 (1963).
Bauer, H., Paronetto, F., Burns, W. A. & Einheber, A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J. Exp. Med. 123, 1013–1024 (1966).
Tsuda, M. et al. Intestinal commensal bacteria promote T cell hyporesponsiveness and down-regulate the serum antibody responses induced by dietary antigen. Immunol. Lett. 132, 45–52 (2010).
Vijay-Kumar, M. et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117, 3909–3921 (2007).
Abraham, C. & Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 140, 1729–1737 (2011).
Macpherson, A. J., McCoy, K. D., Johansen, F. E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Jackson, R. J., Smith, S. D., Wadowsky, R. M., DePudyt, L. & Rowe, M. I. The effect of E coli virulence on bacterial translocation and systemic sepsis in the neonatal rabbit model. J. Pediatr. Surg. 26, 483–485 (1991).
Mahjoub-Messai, F. et al. Escherichia coli isolates causing bacteremia via gut translocation and urinary tract Infection in young infants exhibit different virulence genotypes. J. Infect. Dis. 203, 1844–1849 (2011).
Katayama, M., Xu, D., Specian, R. D. & Deitch, E. A. Role of bacterial adherence and the mucus barrier on bacterial translocation: effects of protein malnutrition and endotoxin in rats. Ann. Surg. 225, 317–326 (1997).
Merlini, E. et al. Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy. PLoS ONE 6, e18580 (2011).
DuPont, H. L. The search for effective treatment of Clostridium difficile infection. N. Engl. J. Med. 364, 473–475 (2011).
Khoruts, A., Dicksved, J., Jansson, J. K. & Sadowsky, M. J. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol. 44, 354–360 (2010).
Grehan, M. J. et al. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J. Clin. Gastroenterol. 44, 551–561 (2010).
Poppe, C. et al. Characterization of antimicrobial resistance of Salmonella Newport isolated from animals, the environment, and animal food products in Canada. Can. J. Vet. Res. 70, 105–114 (2006).
Varma, J. K. et al. Highly resistant Salmonella Newport-MDRAmpC transmitted through the domestic US food supply: a FoodNet case-control study of sporadic Salmonella Newport infections, 2002–2003. J. Infect. Dis. 194, 222–230 (2006).
Neill, M. A. et al. Failure of ciprofloxacin to eradicate convalescent fecal excretion after acute salmonellosis: experience during an outbreak in health care workers. Ann. Intern. Med. 114, 195–199 (1991).
Effler, P. et al. Sporadic Campylobacter jejuni infections in Hawaii: associations with prior antibiotic use and commercially prepared chicken. J. Infect. Dis. 183, 1152–1155 (2001).
Moore, J. E., McLernon, P., Wareing, D., Xu, J. & Murphy, P. G. Characterisation of fluoroquinolone-resistant Campylobacter species isolated from human beings and chickens. Vet. Rec. 150, 518–520 (2002).
Dibaise, J. K., Young, R. J. & Vanderhoof, J. A. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin. Gastroenterol. Hepatol. 4, 11–20 (2006).
Corazza, G. R. et al. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology 98, 302–309 (1990).
Riordan, S. M., McIver, C. J., Duncombe, V. M. & Bolin, T. D. Bacteriologic analysis of mucosal biopsy specimens for detecting small-intestinal bacterial overgrowth. Scand. J. Gastroenterol. 30, 681–685 (1995).
Bratten, J. R., Spanier, J. & Jones, M. P. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am. J. Gastroenterol. 103, 958–963 (2008).
Sahakian, A. B., Jee, S. R. & Pimentel, M. Methane and the gastrointestinal tract. Dig. Dis. Sci. 55, 2135–2143 (2010).
Khoshini, R., Dai, S. C., Lezcano, S. & Pimentel, M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig. Dis. Sci. 53, 1443–1454 (2008).
Bauer, T. M. et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am. J. Gastroenterol. 97, 2364–2370 (2002).
Morencos, F. C. et al. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig. Dis. Sci. 41, 552–556 (1996).
Gunnarsdottir, S. A. et al. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am. J. Gastroenterol. 98, 1362–1370 (2003).
Morencos, F. C. et al. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig. Dis. Sci. 40, 1252–1256 (1995).
Gupta, A. et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J. Hepatol. 53, 849–855 (2010).
Pande, C., Kumar, A. & Sarin, S. K. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment. Pharmacol. Ther. 29, 1273–1281 (2009).
Bjarnason, I., Peters, T. J. & Wise, R. J. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1, 179–182 (1984).
Lorenzo-Zuniga, V. et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 37, 551–557 (2003).
Campillo, B. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur. J. Gastroenterol. Hepatol. 11, 755–759 (1999).
Parlesak, A., Schafer, C., Schutz, T., Bode, J. C. & Bode, C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 32, 742–747 (2000).
Sanchez, E., Casafont, F., Guerra, A., de Benito, I. & Pons-Romero, F. Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation in experimental cirrhosis. Rev. Esp. Enferm. Dig. 97, 805–814 (2005).
Drossman, D. A., Camilleri, M., Mayer, E. A. & Whitehead, W. E. AGA technical review on irritable bowel syndrome. Gastroenterology 123, 2108–2131 (2002).
Kassinen, A. et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133, 24–33 (2007).
Krogius-Kurikka, L. et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 9, 95 (2009).
Lyra, A. et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J. Gastroenterol. 15, 5936–5945 (2009).
Tana, C. et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 22, 512–519, e114–e3115 (2010).
McKernan, D. P., Gaszner, G., Quigley, E. M., Cryan, J. F. & Dinan, T. G. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 33, 1045–1052 (2011).
Schoepfer, A. M., Schaffer, T., Seibold-Schmid, B., Muller, S. & Seibold, F. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol. Motil. 20, 1110–1118 (2008).
Spiller, R. & Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 136, 1979–1988 (2009).
Lee, K. J. & Tack, J. Altered intestinal microbiota in irritable bowel syndrome. Neurogastroenterol. Motil. 22, 493–498 (2010).
Esposito, I. et al. Breath test for differential diagnosis between small intestinal bacterial overgrowth and irritable bowel disease: an observation on non-absorbable antibiotics. World J. Gastroenterol. 13, 6016–6021 (2007).
Lupascu, A. et al. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case–control study in irritable bowel syndrome. Aliment. Pharmacol. Ther. 22, 1157–1160 (2005).
Pimentel, M., Chow, E. J. & Lin, H. C. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 95, 3503–3506 (2000).
Shah, E. D., Basseri, R. J., Chong, K. & Pimentel, M. Abnormal breath testing in IBS: a meta-analysis. Dig. Dis. Sci. 55, 2441–2449 (2010).
Posserud, I., Stotzer, P. O., Bjornsson, E. S., Abrahamsson, H. & Simren, M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 56, 802–808 (2007).
Pimentel, M. et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 364, 22–32 (2011).
Brown, E. L., Xue, Q., Jiang, Z. D., Xu, Y. & Dupont, H. L. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob. Agents Chemother. 54, 388–396 (2010).
Kerlin, P. & Phillips, S. Variability of motility of the ileum and jejunum in healthy humans. Gastroenterology 82, 694–700 (1982).
Vantrappen, G., Janssens, J., Hellemans, J. & Ghoos, Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J. Clin. Invest. 59, 1158–1166 (1977).
Pimentel, M., Soffer, E. E., Chow, E. J., Kong, Y. & Lin, H. C. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig. Dis. Sci. 47, 2639–2643 (2002).
Barbara, G. et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 20 (Suppl. 2), 1–9 (2004).
Lin, H. C. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA 292, 852–858 (2004).
Salonen, A., de Vos, W. M. & Palva, A. Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology 156, 3205–3215 (2010).
Andoh, A. et al. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn's disease using terminal restriction fragment length polymorphism analysis. J. Gastroenterol. 46, 479–486 (2011).
Manichanh, C. et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55, 205–211 (2006).
Ott, S. J. et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53, 685–693 (2004).
Onderdonk, A. B., Hermos, J. A. & Bartlett, J. G. The role of the intestinal microflora in experimental colitis. Am. J. Clin. Nutr. 30, 1819–1825 (1977).
Lal, S. & Steinhart, A. H. Antibiotic therapy for Crohn's disease: a review. Can. J. Gastroenterol. 20, 651–655 (2006).
Rutgeerts, P. et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet 338, 771–774 (1991).
Mow, W. S. et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology 126, 414–424 (2004).
Sartor, R. B. Microbial influences in inflammatory bowel diseases. Gastroenterology 134, 577–594 (2008).
Martin, H. M. et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 127, 80–93 (2004).
Subramanian, S. et al. Characterization of epithelial IL-8 response to inflammatory bowel disease mucosal, E. coli and its inhibition by mesalamine. Inflamm. Bowel Dis. 14, 162–175 (2008).
Swidsinski, A. et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 122, 44–54 (2002).
Chassaing, B. & Darfeuille-Michaud, A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140, 1720–1728 e3 (2011).
Gewirtz, A. T. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr. Opin. Gastroenterol. 22, 8–12 (2006).
Wullaert, A. Role of NF-kappaB activation in intestinal immune homeostasis. Int. J. Med. Microbiol. 300, 49–56 (2010).
Pruteanu, M., Hyland, N. P., Clarke, D. J., Kiely, B. & Shanahan, F. Degradation of the extracellular matrix components by bacterial-derived metalloproteases: implications for inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 1189–1200 (2010).
Maccaferri, S. et al. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J. Antimicrob. Chemother. 65, 2556–2565 (2010).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Ott, S. J. & Schreiber, S. Reduced microbial diversity in inflammatory bowel diseases. Gut 55, 1207 (2006).
Mondot, S. et al. Highlighting new phylogenetic specificities of Crohn's disease microbiota. Inflamm. Bowel Dis. 17, 185–192 (2011).
Bibiloni, R., Mangold, M., Madsen, K. L., Fedorak, R. N. & Tannock, G. W. The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn's disease and ulcerative colitis patients. J. Med. Microbiol. 55, 1141–1149 (2006).
Seksik, P. et al. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 52, 237–242 (2003).
Chassaing, B. et al. Crohn disease--associated adherent-invasive, E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. J. Clin. Invest. 121, 966–975 (2011).
Darfeuille-Michaud, A. et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127, 412–421 (2004).
Jia, W. et al. Is the abundance of Faecalibacterium prausnitzii relevant to Crohn's disease? FEMS Microbiol. Lett. 310, 138–144 (2010).
Willing, B. et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm. Bowel Dis. 15. 653–660 (2009).
Schippa, S. et al. Dominant genotypes in mucosa-associated Escherichia coli strains from pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 15, 661–672 (2009).
Petersen, A. M. et al. A phylogenetic group of Escherichia coli associated with active left-sided inflammatory bowel disease. BMC Microbiol. 9, 171 (2009).
Mitchell, D. N. & Rees, R. J. Agent transmissible from Crohn's disease tissue. Lancet 2, 168–171 (1970).
Dessein, R., Rosenstiel, P. & Chamaillard, M. Debugging the intestinal microbiota in IBD. Gastroenterol. Clin. Biol. 33 (Suppl. 3), S131–S136 (2009).
Hollander, D. et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 105, 883–885 (1986).
Keita, A. V. et al. Increased uptake of non-pathogenic, E. coli via the follicle-associated epithelium in longstanding ileal Crohn's disease. J. Pathol. 215, 135–144 (2008).
Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Swidsinski, A., Weber, J., Loening-Baucke, V., Hale, L. P. & Lochs, H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 43, 3380–3389 (2005).
Achkar, J. P. & Duerr, R. The expanding universe of inflammatory bowel disease genetics. Curr. Opin. Gastroenterol. 24, 429–434 (2008).
Sydora, B. C., McFarlane, S. M., Doyle, J. S. & Fedorak, R. N. Neonatal exposure to fecal antigens reduces intestinal inflammation. Inflamm. Bowel Dis. 17, 899–906 (2011).
Singhal, S. et al. The role of oral hygiene in inflammatory bowel disease. Dig. Dis. Sci. 56, 170–175 (2011).
Matricon, J., Barnich, N. & Ardid, D. Immunopathogenesis of inflammatory bowel disease. Self Nonself 1, 299–309 (2010).
Sobhani, I. et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 6, e16393 (2011).
Swidsinski, A. et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology 115, 281–286 (1998).
de Martel, C. & Franceschi, S. Infections and cancer: established associations and new hypotheses. Crit. Rev. Oncol. Hematol. 70, 183–194 (2009).
Chung, K. T., Stevens, S. E. Jr & Cerniglia, C. E. The reduction of azo dyes by the intestinal microflora. Crit. Rev. Microbiol. 18, 175–190 (1992).
Walker, A. W. et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5, 220–230 (2010).
Candela, M. et al. Human intestinal microbiota: cross-talk with the host and its potential role in colorectal cancer. Crit. Rev. Microbiol. 37, 1–14 (2011).
Breuer, N. & Goebell, H. The role of bile acids in colonic carcinogenesis. Klin. Wochenschr. 63, 97–105 (1985).
Hope, M. E., Hold, G. L., Kain, R. & El-Omar, E. M. Sporadic colorectal cancer—role of the commensal microbiota. FEMS Microbiol. Lett. 244, 1–7 (2005).
Zhang, M. M., Cheng, J. Q., Xia, L., Lu, Y. R. & Wu, X. T. Monitoring intestinal microbiota profile: a promising method for the ultraearly detection of colorectal cancer. Med. Hypotheses 76, 670–672 (2011).
Fallani, M. et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157, 1385–1392 (2011).
Shen, Q., Chen, Y. A., Tuohy, K. M. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe 16, 572–577 (2010).
Vulevic, J., Rastall, R. A. & Gibson, G. R. Developing a quantitative approach for determining the in vitro prebiotic potential of dietary oligosaccharides. FEMS Microbiol. Lett. 236, 153–159 (2004).
Bodera, P. Influence of prebiotics on the human immune system (GALT). Recent Pat. Inflamm. Allergy Drug Discov. 2, 149–153 (2008).
Schley, P. D. & Field, C. J. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 87 (Suppl. 2), S221–S230 (2002).
Pothoulakis, C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 30, 826–833 (2009).
D'Inca, R. et al. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig. Dis. Sci. 56, 1178–1187 (2011).
Gareau, M. G., Sherman, P. M. & Walker, W. A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 7, 503–514 (2010).
Oelschlaeger, T. A. Mechanisms of probiotic actions—a review. Int. J. Med. Microbiol. 300, 57–62 (2010).
Damaskos, D. & Kolios, G. Probiotics and prebiotics in inflammatory bowel disease: microflora 'on the scope'. Br. J. Clin. Pharmacol. 65, 453–467 (2008).
Silverman, M. S., Davis, I. & Pillai, D. R. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 8, 471–473 (2010).
Sproule-Willoughby, K. M. et al. In vitro anaerobic biofilms of human colonic microbiota. J. Microbiol. Methods 83, 296–301 (2010).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
A. W. DuPont receives grant/research support from Salix Pharmaceuticals and is a consultant for Lexicon Pharmaceuticals. H. L. DuPont receives grant/research support from Intercell, Osel Inc., Santarus and Novartis. He acts as a consultant, is on the speakers bureau and receives grant/research support from Salix Pharmaceuticals.
Rights and permissions
About this article
Cite this article
DuPont, A., DuPont, H. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 8, 523–531 (2011). https://doi.org/10.1038/nrgastro.2011.133
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.133
This article is cited by
-
Endogenous small intestinal microbiome determinants of transient colonisation efficiency by bacteria from fermented dairy products: a randomised controlled trial
Microbiome (2023)
-
Lactobacillus plantarum strains attenuated DSS-induced colitis in mice by modulating the gut microbiota and immune response
International Microbiology (2022)
-
Individual fate and gut microbiome composition in the European wild rabbit (Oryctolagus cuniculus)
Scientific Reports (2021)
-
Putative Probiotic Strains Isolated from Kefir Improve Gastrointestinal Health Parameters in Adults: a Randomized, Single-Blind, Placebo-Controlled Study
Probiotics and Antimicrobial Proteins (2020)
-
Microbiota of Four Tissue Types in American Alligators (Alligator mississippiensis) Following Extended Dietary Selenomethionine Exposure
Bulletin of Environmental Contamination and Toxicology (2020)