Abstract
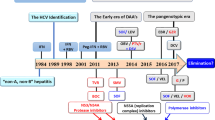
Therapy for hepatitis C has been fairly stagnant for the past decade, but the past few years have seen major progress and evolution, beginning with the approval of two HCV protease inhibitors in 2011. In spite of considerable improvements in response rates with these agents, a need for additional agents with improved potency and tolerability remains. Toward this goal and over the course of just a few months, the HCV therapy pipeline has already become crowded with direct-acting antivirals, host-targeted agents and unique interferons, all of which are positioned to be part of the next wave of therapeutic options. The ultimate goal of this push for new agents is to achieve a safe and straight forward yet highly effective therapy for hepatitis C that is widely embraced and readily available. Particularly among the 'baby boomer' population, it is predicted that over the next few years, more patients with currently quiescent infections will be newly diagnosed, and those currently diagnosed will be at increased risk of long-term complications of infection, and thus in need of treatment. A simple and safe treatment paradigm will become a necessity. This Review chronicles the latest developments in hepatitis C therapy and the potential effect these new treatments could have on delivery of care to patients infected with HCV.
Key Points
-
Continued investigation into direct-acting antiviral therapy, new host factor targets and novel interferons for HCV is proceeding rapidly and should lead to simpler therapies with improved cure rates soon
-
People born 1945–1964 comprise a large proportion of infected patients (mostly undiagnosed) and are targeted for screening in new initiatives recommended by the Centers for Disease Control and Prevention
-
In the USA, an estimated 800,000 new patients with HCV will be diagnosed through this plan in the next few years, further increasing the need for an effective linkage to care
-
A goal of future therapies for HCV is ideally an interferon-free, short-duration, highly effective and well-tolerated treatment to expand the pool of treated patients
-
New therapies with improved safety and effectiveness will hopefully also enable a broader treatment-delivery cohort—including gastroenterologists, primary care physicians and infectious disease specialists—to treat the increasing population of patients
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
07 August 2013
In the version of this article originally published online and in print, a finding was listed incorrectly in Table 6. The error has been corrected for the HTML and PDF versions of the article.
References
WHO. Hepatitis C: WHO Fact sheet N° 164 [online], (2013).
Lavanchy, D. The global burden of hepatitis C. Liver Int. 29, 74–81 (2009).
U. S. Department of Health and Human Services: Centers for Disease Control and Prevention. The ABCs of hepatitis [online], (2012).
Smith, B. D. Morbidity and mortality weekly report: Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. Centers for Disease Control and Prevention [online], (2012).
Micallef, J. M., Kaldor, J. M. & Dore, G. J. Spontaneous viral clearance following acute hepatitis C infection: A systematic review of longitudinal studies. J. Viral Hepat. 13, 34–41 (2006).
McHutchison, J. G. & Bacon, B. R. Chronic hepatitis C: An age wave of disease burden. Am. J. Manag. Care 11 (10 Suppl.), S286–S295 (2005).
El-Serag, H. B. Epidemiology of hepatitis C-related hepatocellular carcinoma: HCV and HCC. Medscape [online], (2007).
El-Serag, H. B. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology 36 (Suppl. 1), S74–S83 (2002).
Dore, G. J., Freeman, A. J., Law, M. & Kaldor, J. Is severe liver disease a common outcome for people with chronic hepatitis C? J. Gastroenterol. Hepatol. 17, 423–430 (2002).
Freeman, A. J. et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 34, 809–816 (2001).
Freeman, R. B. Jr et al. Liver and intestine transplantation in the United States, 1997–2006. Am. J. Transplant. 8, 958–976 (2008).
Sanyal, A. J., Governing Board the Public Policy, Clinical Practice, Manpower committees of the AASLD. The institute of medicine report on viral hepatitis: A call to action. Hepatology 51, 727–728 (2010).
Velazquez, R. F. et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 37, 520–527 (2003).
Kanwal, F. et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 140, 1182–1188.e1 (2011).
Pearlman, B. L. & Traub, N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: A cure and so much more. Clin. Infect. Dis. 52, 889–900 (2011).
Seeff, L. B. Sustained virologic response: Is this equivalent to cure of chronic hepatitis C? Hepatology 57, 438–440 (2013).
Poynard, T., Moussalli, J., Ratziu, V., Regimbeau, C. & Opolon, P. Effect of interferon therapy on the natural history of hepatitis C virus-related cirrhosis and hepatocellular carcinoma. Clin. Liver Dis. 3, 869–881 (1999).
Kagawa, T. & Keeffe, E. B. Long-term effects of antiviral therapy in patients with chronic hepatitis C. Hepat. Res. Treat. 2010, 562578 (2010).
Yoshida, H. et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 123, 483–491 (2002).
Aronsohn, A. & Reau, N. Long-term outcomes after treatment with interferon and ribavirin in HCV patients. J. Clin. Gastroenterol. 43, 661–671 (2009).
Grebely, J. & Dore, G. J. What is killing people with hepatitis C virus infection? Semin. Liver Dis. 31, 331–339 (2011).
Pyenson, B., Fitch, K. & Iwasaki, K. Consequences of hepatitis C virus (HCV): Costs of a baby boomer epidemic of liver disease. Milliman [online], (2009).
Manns, M. P. et al. Peginterferon α-2b plus ribavirin compared with interferon α-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 358, 958–965 (2001).
Fried, M. W. et al. Peginterferon α-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347, 975–982 (2002).
Zeuzem, S. Interferon-based therapy for chronic hepatitis C: Current and future perspectives. Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 610–622 (2008).
Muir, A. J., Bornstein, J. D. & Killenberg, P. G., Atlantic Coast Hepatitis Treatment Group. Peginterferon α-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-hispanic whites. N. Engl. J. Med. 350, 2265–2271 (2004).
Bacon, B. R. & McHutchison, J. G. Treatment issues with chronic hepatitis C: Special populations and pharmacy strategies. Am. J. Manag. Care 11 (10 Suppl.), S296–S306 (2005).
Kinzie, J. L. et al. African Americans with genotype 1 treated with interferon for chronic hepatitis C have a lower end of treatment response than Caucasians. J. Viral Hepat. 8, 264–269 (2001).
Hayes, C. N., Imamura, M., Aikata, H. & Chayama, K. Genetics of IL28B and HCV-response to infection and treatment. Nat. Rev. Gastroenterol. Hepatol. 9, 406–417 (2012).
Bacon, B. R. et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 364, 1207–1217 (2011).
Poordad, F. et al. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364, 1195–1206 (2011).
McHutchison, J. G. et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N. Engl. J. Med. 360, 1827–1838 (2009).
Jacobson, I. M. et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364, 2405–2416 (2011).
Schaefer, E. A. & Chung, R. T. Anti-hepatitis C virus drugs in development. Gastroenterology 142, 1340–1350 (2012).
Jensen, D. M. A new era of hepatitis C therapy begins. N. Engl. J. Med. 364, 1272–1274 (2011).
Zeuzem, S. et al. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 364, 2417–2428 (2011).
Boehringer Ingelheim. First ever data investigating interferon-free treatment in hepatitis C patients who have liver cirrhosis shows high viral cure rate [online], (2012).
Schalm, S. W. et al. Interferon-ribavirin for chronic hepatitis C with and without cirrhosis: Analysis of individual patient data of six controlled trials. Eurohep study group for viral hepatitis. Gastroenterology 117, 408–413 (1999).
Idilman, R., De Maria, N., Colantoni, A., Dokmeci, A. & Van Thiel, D. H. Interferon treatment of cirrhotic patients with chronic hepatitis C. J. Viral Hepat. 4, 81–91 (1997).
Ward, R. P., Kugelmas, M. & Libsch, K. D. Management of hepatitis C: Evaluating suitability for drug therapy. Am. Fam. Physician 69, 1429–1436 (2004).
Zeuzem, S. et al. The ASPIRE trial: TMC435 in treatment-experienced patients with genotype-1 HCV infection who have failed previous PEGIFN/RBV treatment [abstract 1376]. J. Hepatol. 54 (Suppl. 1), S546 (2011).
Zeuzem, S. et al. TMC435 in HCV genotype 1 patients who have failed previous pegylated interferon/ribavirin treatment: final SVR24 results of the ASPIRE trial [abstract 2]. J. Hepatol. 56 (Suppl. 2), S1–S2 (2012).
Sulkowski, M. et al. Impact of early response definitions on duration and outcome of treatment with BI201335 plus PR [abstract 1209]. J. Hepatol. 56 (Suppl. 2), S479–S480 (2012).
Sulkowski, M. S. et al. Treatment with the second generation HCV protease inhibitor BI201335 results in high and consistent SVR rates—results from SILEN-C1 in treatment naïve patients across different baseline factors [abstract 226]. Hepatol. 54 (Suppl.), 473A (2011).
Sulkowski, M. et al. SILEN-C2: Sustained virologic response and safety of BI-201335 combined with peginterferon α-2a and ribavirin (P/R) in chronic HCV genotype 1 patients with non-response to P/R [abstract 66]. J. Hepatol. 54 (Suppl. 1), 30 (2011).
Fridell, R. A. et al. Distinct functions of NS5A in hepatitis C virus RNA replication uncovered by studies with the NS5A inhibitor BMS-790052. J. Virol. 85, 7312–7320 (2011).
Lin, H. M. et al. Resistance analysis and characterization of a thiazole analogue, BP008, as a potent hepatitis C virus NS5A inhibitor. Antimicrob. Agents Chemother. 56, 44–53 (2012).
Gao, M. et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465, 96–100 (2010).
Nettles, R. E. et al. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 54, 1956–1965 (2011).
Lawitz, E. et al. Safety and antiviral activity of ABT-267, a novel NS5A inhibitor, during 3-day monotherapy: First study in HCV genotype-1 (GT1)-infected treatment-naïve subjects [abstract 1186]. J. Hepatol. 56 (Suppl. 2), S469–S470 (2012).
Pawlotsky, J. M. Hepatitis C virus genetic variability: Pathogenic and clinical implications. Clin. Liver Dis. 7, 45–66 (2003).
Simmonds, P. Genetic diversity and evolution of hepatitis C virus--15 years on. J. Gen. Virol. 85, 3173–3188 (2004).
Powdrill, M. H., Bernatchez, J. A. & Gotte, M. Inhibitors of the hepatitis C virus RNA-dependent RNA polymerase NS5B. Viruses 2, 2169–2195 (2010).
Rodriguez-Torres, M. et al. HIV/HCV coinfected and HCV monoinfected patients have similar early HCV viral kinetics with the potent HCV nucleotide polymerase inhibitor sofosbuvir (SOF) [abstract H1921a]. Presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy.
Gane, E. J. et al. Electron: Once daily PSI-7977 plus RBV in HCV GT1/2/3 [abstract 1113]. J. Hepatol. 56, S438–S439 (2012).
Gane, E. J. et al. Once daily sofosbuvir (GS-7977) regimens in HCV genotype 1–3: The ELECTRON trial. Presented at the American Association for the Study of Liver Diseases, 2012.
Le Pogam, S. et al. RG7128 alone or in combination with pegylated interferon-α2a and ribavirin prevents hepatitis C virus (HCV) replication and selection of resistant variants in HCV-infected patients. J. Infect. Dis. 202, 1510–1519 (2010).
Jensen, D. M. et al. High rates of early viral response, promising safety profile and lack of resistance-related breakthrough in HCV GT 1/4 patients treated with RG7128 plus PEGIFN α-2a/RBV: Planned week 12 interim analysis from the PROPEL study. Presented at the American Association for the Study of Liver Diseases, 2010.
Pockros, P. J. et al. JUMP-C: A randomized trial of mericitabine plus peginterferon α-2a/ribavirin for 24 weeks in treatment-naive HCV genotype 1/4 patients. Hepatology http://dx.doi.org/10.1002/hep.26275.
Pockros, P. et al. First SVR data with the nucleoside analogue polymerase inhibitor mericitabine (RG7128) combined with peginterferon/ribavirin in treatment-naive HCV G1/4 patients: Interim analysis from the JUMP-C trial. Presented at the 46th European Association for the Study of the Liver Congress.
Pollack, A. Bristol-Myers ends a hepatitis C project. New York Times (23 August 2012). Available from: http://www.nytimes.com/2012/08/24/business/bristol-myers-ends-work-on-hepatitis-c-drug.html.
Jacobson, I. M. et al. Virologic response rates following 4 weeks of filibuvir in combination with pegylated interferon α-2a and ribavirin in chronically-infected HCV genotype-1 patients [abstract 2005]. J. Hepatol. 52 (Suppl.), S465 (2010).
Flisiak, R. et al. The cyclophilin inhibitor debio 025 combined with PEGIFN α2a significantly reduces viral load in treatment-naive hepatitis C patients. Hepatology 49, 1460–1468 (2009).
Flisiak, R. et al. Once daily alisporivir (DEB025) plus PegIFNα2a/ribavirin results in superior sustained virologic response (SVR24) in chronic hepatitis C genotype 1 treatment naive patients. Presented at the 46th Annual Meeting of the European Association for the Study of the Liver.
Li, B. et al. Alisporivir—a host-targeting antiviral provides low viral breakthrough rate and high barrier to resistance in HCV genotype 1 treatment-naive patients in the phase IIb ESSENTIAL study. Presented at the 62nd Annual Meeting of the American Association for the Study of Liver Diseases.
Copley, C. FDA puts Novartis DEB025 clinical trial on hold. Reuters [online], (2012).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009).
Bristol-Myers Squibb. Investigational compound PEG-interferon λ achieved higher response rates with fewer flu-like and musculoskeletal symptoms and cytopenias than PEG-interferon α in phase IIb study of 526 treatment-naive hepatitis C patients. Bristol-Myers Squibb [online], (2011).
Zeuzem, S. et al. Pegylated interferon-λ (pegIFN-λ) shows superior viral response with improved safety and tolerability versus pegIFN-α-2a in HCV patients (G1/2/3/4): EMERGE phase IIb through week 12 [abstract 422]. Presented at the 46th Annual Meeting of the European Association for the Study of the Liver.
Muir, A. et al. Peginterferon λ compared with peginterferon α in treatment-naive patients with HCV genotypes 1 or 4: SVR24 results from EMERGE phase 2b. Presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases.
Loftus, P. Pharmasset halts hepatitis C drug in test. The Wall Street Journal (16 December, 2011). Available from: http://online.wsj.com/article/SB10001424052970204553904577102383041237416.html.
Deniz, B., Pyenson, B., Iwasaki, K. & Graham, C. S. Hepatitis C virus (HCV) testing and diagnosis rates in the commercially insured and medicare populations in the U. S. 2011, October 31. Presented at the 33rd International Conference on Viral Hepatitis.
Mitchell, A. E., Colvin, H. M. & Palmer Beasley, R. Institute of medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 51, 729–733 (2010).
CDC. Morbidity and mortality weekly report: Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease [online], (1998).
U. S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA). 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus [online], (1999).
Craxi, A. et al. Third-generation hepatitis C virus tests in asymptomatic anti-HCV-positive blood donors. J. Hepatol. 21, 730–734 (1994).
Ghany, M. G. et al. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 49, 1335–1374 (2009).
McGowan, C. E. et al. A global view of hepatitis C: Physician knowledge, opinions, and perceived barriers to care. Hepatology http://dx.doi.org/10.1002/hep.26246.
Reesink, H. W. et al. Rapid HCV-RNA decline with once daily TMC435: A phase I study in healthy volunteers and hepatitis C patients. Gastroenterology 138, 913–921 (2010).
Welsch, C. & Zeuzem, S. Will interferon-free regimens prevail? Gastroenterology 142, 1351–1355 (2012).
Vierling, J. et al. Efficacy of boceprevir in prior null responders to peginterferon/ribavirin: The PROVIDE study. Presented at the 62nd Annual Meeting of the American Association for the Study of Liver Diseases.
Fried, M. W. et al. TMC435 in combination with peginterferon and ribavarin in treatment-naive HCV genotype 1 patients: Final analysis of the PILLAR phase IIB study. Hepatology 52 (4 Suppl.), LB-5 (2010).
Sulkowski, M. et al. High sustained virologic response rate in treatment-naive HCV genotype 1a and 1b patients treated for 12 weeks with an interferon-free all-oral quad regimen: Interim results [abstract 1421]. J. Hepatol. 56, S560 (2012).
Zeuzem, S. F. et al. Efficacy of the protease inhibitor BI 201335, polymerase inhibitor BI 207127, and ribavirin in patients with chronic HCV infection. Gastroenterology 141, 2047–2055 (2011).
Soriano, V. et al. The efficacy and safety of the interferon-free combination of BI 201335 and BI 207127 in genotype 1 HCV patients with cirrhosis: Interim analysis from SOUND-C2 [abstract 1420]. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver.
Zeuzem, S. et al. SVR4 and SVR12 with an interferon-free regimen of BI 201335 and BI 207127, ± ribavirin, in treatment-naive patients with chronic genotype-1 HCV infection: Interim results of SOUND-C2 [abstract 101]. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver.
Poordad, F. et al. 12-week interferon-free regimen of ABT-450/R +ABT-333 +ribavirin achieved SVR12 in more than 90% of treatment-naive HCV genotype-1-infected subjects and 47% of previous non-responders [abstract 1339]. J. Hepatol. 56, S549–S550 (2012).
Lawitz, E. et al. A 12-week interferon-free regimen of ABT-450/R, ABT-072, and ribavirin was well tolerated and achieved sustained virologic response in 91% treatment-naive HCV IL28B-CC genotype-1-infected subjects [abstract 13]. J. Hepatol. 56, S7 (2012).
Gane, E. J. et al. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG-IFN I±-2a/RBV in hepatitis C patients. J. Hepatol. 55, 972–979 (2011).
Gane, E. J. et al. Interferon-free treatment with a combination of mericitabine and danoprevir/r with or without ribavirin in treatment-naive HCV genotype 1-infected patients [abstract 1412]. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver.
Pawlotsky, J. M. et al. Alisporivir plus ribavirin is highly effective as interferon-free or interferon-add-on regimen in previously untreated HCV-GT2 or GT3 patients: SVR12 results from VITAL-1 phase 2b study [abstract 1405]. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver.
Kowdley, K. et al. A 12-week interferon-free treatment regimen with ABT450/r, ABT267, ABT333 and ribavirin achieves SVR12 rates of 99% in treatment-naïve patients and 93% in prior null responders with HCV genotype 1 infection. Presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases.
Everson, G. T. et al. An interferon-free, ribavirin-free 12-week regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 yielded SVR4 of 94% in treatment-naïve patients with genotype (GT) 1 chronic hepatitis C virus (HCV) infection. Presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases.
Lok, A. S. et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366, 216–224 (2012).
Feld, J. J. et al. Up to 100% SVR4 rates with ritonavir-boosted danoprevir (DNVr), mericitabine and ribavirin with or without peginterferon α-2a (40KD) in HCV genotype 1-infected partial and null responders: Results from the MATTERHORN study. Presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases.
Terrault, N. et al. High sustained virologic response (SVR24) rates with response-guided danoprevir (DNV; RG7227) plus PegIFN α-2a (40KD) and ribavirin (P/R) in treatment-naive HCV genotype 1 (G1) patients: Results from the ATLAS study. Hepatology 54, 398A (2011).
Vierling, J. et al. Once daily narlaprevir (SCH 900518) in combination with peginterferon alfa-2b/ ribavirin for treatment-naive patients with genotype-1 chronic hepatitis C: Interim results from the NEXT-1 study. Presented at the 60th Annual Meeting of the American Association for the Study of Liver Diseases.
Manns, M. et al. Sustained viral response (SVR) rates in genotype 1 treatment-naïve patients with chronic hepatitis C (CHC) infection treated with vaniprevir (MK-7009), a NS3/4a protease inhibitor, in combination with pegylated interferon α-2a and ribavirin for 28 days. Presented at the 61st Annual Meeting of the American Association for the Study of Liver Diseases.
Lawitz, E. et al. 1187 ABT-450/ritonavir (ABT-450/R) combined with pegylated interferon α-2a/ribavirin after 3-day monotherapy in genotype 1 (gt1) HCV-infected treatment-naive subjects: 12-week sustained virologic response (SVR12) and safety results. J. Hepatol. 56, 470 (2012).
Bronowicki, J. et al. Asunaprevir (ASV; BMS-650032), an NS3 protease inhibitor, in combination with peginterferon and ribavirin in treatment-naive patients with genotype 1 chronic hepatitis C infection. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver.
Pol, S. et al. High rates of SVR24 for BMS-790052, an NS5A replication complex inhibitor, in combination with PegIFN-alfa-2a and ribavirin: Phase 2a trial in treatment-naive HCV genotype 1 subjects [oral abstract H1–376]. Presented at the 51st Interscience Conference on Antimicrobioal Agents and Chemotherapy.
D-LITE study group. Bristol-Myers Squibb's investigational hepatitis C compounds lambda and daclatasvir plus ribavirin achieved SVR12 in 93% of genotype 1b treatment-naive patients in phase IIb study. Presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases.
Lawitz, E. et al. PROTON: PSI-7977 & Peg/RBV in treatment-naïve patients with HCV GT1: Sustained virologic response. Presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases.
Kowdlev, K. V. et al. 1 ATOMIC: 97% RVR for PSI-7977 + PEG/RBV A—12 week regimen in HCV GT1: an end to response-guided therapy? J. Hepatol. 56, 1 (2012).
Flisiak, R. et al. Alisporivir (ALV) plus peg-IFN/RBV (PR) has 100% SVR in IL28B rs12979860 CC allele and superior efficacy in chronic hepatitis C genotype (G) 1 treatment-naive patients compared with PR: the essential study. Presented at the 22nd Conference of the Asian Pacific Association for the Study of the Liver.
Gane, E. J. et al. VX-222/telaprevir in combination with peginterferon-α-2a and ribavirin in treatment-naïve genotype 1 HCV patients treated for 12 weeks: ZENITH study, SVR12 interim analysis. Presented at the 22nd Conference of the Asian Pacific Association for the Study of the Liver.
Nelson, D. R. et al. High SVR12 with 16 weeks of tegobuvir and GS-9256 with peginterferon-α 2a and ribavirin in treatment-naive genotype 1 HCV patients. J. Hepatol. 56, S6–S7 (2012).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
D. M. Jensen is a consultant for Abbott, Astex, Biotica, Boehringer-Ingelheim, Bristol Myers Squibb, Genentech, Gilead, Janssen, Merck and Vertex and a clinical research investigator for Abbott, Boehringer-Ingelheim, Bristol Myers Squibb, Genentech, Gilead and Janssen. N. M. Dabbouseh declares no competing interests.
Rights and permissions
About this article
Cite this article
Dabbouseh, N., Jensen, D. Future therapies for chronic hepatitis C. Nat Rev Gastroenterol Hepatol 10, 268–276 (2013). https://doi.org/10.1038/nrgastro.2013.17
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.17
This article is cited by
-
Chemopreventive strategies in hepatocellular carcinoma
Nature Reviews Gastroenterology & Hepatology (2014)
-
HCV transmission in industrialized countries and resource-constrained areas
Nature Reviews Gastroenterology & Hepatology (2014)
-
Discovering novel anti-HCV compounds with inhibitory activities toward HCV NS3/4A protease
Acta Pharmacologica Sinica (2014)
-
Functional brain imaging in gastroenterology: to new beginnings
Nature Reviews Gastroenterology & Hepatology (2014)
-
The Acyclic Retinoid Peretinoin Inhibits Hepatitis C Virus Replication and Infectious Virus Release in Vitro
Scientific Reports (2014)