Key Points
-
Gastrointestinal infection and inflammation are key risk factors for the development of numerous clinical gastrointestinal disorders that present with symptoms such as altered motility or secretion, abdominal discomfort and pain
-
Neuronal processing along the gut–brain axis is crucial for the function and modulation of key gastrointestinal processes; findings suggest that this processing can be altered by gut inflammation or infection
-
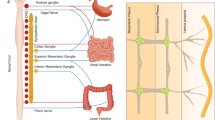
Inflammation or infection causes specific changes in enteric neuronal excitability, which can persist after inflammation has resolved; in some experimental models, inflammation also causes a rapid loss of myenteric neurons and viscerofugal neurons
-
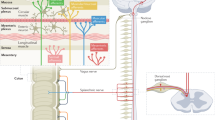
Inflammation causes a specific hypersensitivity of visceromotor sympathetic neurons in prevertebral ganglia, which persists long after inflammation has resolved
-
Extrinsic gut sensory afferents express pronociceptive channels and receptors that can be activated in response to inflammatory and immune mediators, leading to acute neuronal hyperexcitability, visceral hypersensitivity and neurogenic inflammation
-
Inflammation causes lowering of mechanical activation thresholds of high-threshold or low-threshold afferents, which leads to hyperexcitability in afferent neuronal cell bodies, and increased activation of nociceptive pathways in the central nervous system
Abstract
The gastrointestinal tract is innervated by several distinct populations of neurons, whose cell bodies either reside within (intrinsic) or outside (extrinsic) the gastrointestinal wall. Normally, most individuals are unaware of the continuous, complicated functions of these neurons. However, for patients with gastrointestinal disorders, such as IBD and IBS, altered gastrointestinal motility, discomfort and pain are common, debilitating symptoms. Although bouts of intestinal inflammation underlie the symptoms associated with IBD, increasing preclinical and clinical evidence indicates that infection and inflammation are also key risk factors for the development of other gastrointestinal disorders. Notably, a strong correlation exists between prior exposure to gut infection and symptom occurrence in IBS. This Review discusses the evidence for neuroplasticity (structural, synaptic or intrinsic changes that alter neuronal function) affecting gastrointestinal function. Such changes are evident during inflammation and, in many cases, long after healing of the damaged tissues, when the nervous system fails to reset back to normal. Neuroplasticity within distinct populations of neurons has a fundamental role in the aberrant motility, secretion and sensation associated with common clinical gastrointestinal disorders. To find appropriate therapeutic treatments for these disorders, the extent and time course of neuroplasticity must be fully appreciated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Kiank, C., Tache, Y. & Larauche, M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav. Immun. 24, 41–48 (2010).
Larauche, M., Mulak, A. & Tache, Y. Stress and visceral pain: from animal models to clinical therapies. Exp. Neurol. 233, 49–67 (2012).
Ohman, L. & Simren, M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 7, 163–173 (2010).
Collins, S. M., Surette, M. & Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 10, 735–742 (2012).
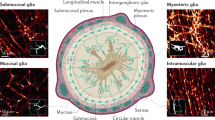
Gulbransen, B. D. & Sharkey, K. A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 9, 625–632 (2012).
Zeltser, L. M., Seeley, R. J. & Tschop, M. H. Synaptic plasticity in neuronal circuits regulating energy balance. Nat. Neurosci. 15, 1336–1342 (2012).
Hoogerwerf, W. A. Role of biological rhythms in gastrointestinal health and disease. Rev. Endocr. Metab. Disord. 10, 293–300 (2009).
Cummings, D. E. & Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Invest. 117, 13–23 (2007).
Baumgart, D. C. & Sandborn, W. J. Crohn's disease. Lancet 380, 1590–1605 (2012).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N. Engl. J. Med. 362, 1383–1395 (2010).
Mantzaris, G. J. et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn's disease. Inflamm. Bowel Dis. 15, 375–382 (2009).
Casellas, F. et al. Mucosal healing restores normal health and quality of life in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 24, 762–769 (2012).
Higgins, P. D. et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 54, 782–788 (2005).
Rao, S. S., Read, N. W., Brown, C., Bruce, C. & Holdsworth, C. D. Studies on the mechanism of bowel disturbance in ulcerative colitis. Gastroenterology 93, 934–940 (1987).
Bassotti, G. et al. Twenty-four-hour manometric study of colonic propulsive activity in patients with diarrhea due to inflammatory (ulcerative colitis) and non-inflammatory (irritable bowel syndrome) conditions. Int. J. Colorectal Dis. 19, 493–497 (2004).
Kern, F. Jr, Almy, T. P., Abbot, F. K. & Bogdonoff, M. D. The motility of the distal colon in nonspecific ulcerative colitis. Gastroenterology 19, 492–503 (1951).
Snape, W. J. Jr, Matarazzo, S. A. & Cohen, S. Abnormal gastrocolonic response in patients with ulcerative colitis. Gut 21, 392–396 (1980).
Koch, T. R., Carney, J. A., Go, V. L. & Szurszewski, J. H. Spontaneous contractions and some electrophysiologic properties of circular muscle from normal sigmoid colon and ulcerative colitis. Gastroenterology 95, 77–84 (1988).
Reddy, S. N. et al. Colonic motility and transit in health and ulcerative colitis. Gastroenterology 101, 1289–1297 (1991).
Odille, F. et al. Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn. Reson. Med. 68, 783–793 (2012).
Tursi, A., Brandimarte, G., Giorgetti, G. & Nasi, G. Assessment of orocaecal transit time in different localization of Crohn's disease and its possible influence on clinical response to therapy. Eur. J. Gastroenterol. Hepatol. 15, 69–74 (2003).
Keller, J., Beglinger, C., Holst, J. J., Andresen, V. & Layer, P. Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G861–G868 (2009).
Nobrega, A. C. et al. Dyspeptic symptoms and delayed gastric emptying of solids in patients with inactive Crohn's disease. BMC Gastroenterol. 12, 175 (2012).
Mueller, M. H. et al. Anorectal functional disorders in the absence of anorectal inflammation in patients with Crohn's disease. Br. J. Surg. 89, 1027–1031 (2002).
Bernstein, C. N. et al. Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain 66, 151–161 (1996).
Chrysos, E. et al. Rectoanal motility in Crohn's disease patients. Dis. Colon Rectum 44, 1509–1513 (2001).
Kangas, E., Hiltunen, K. M. & Matikainen, M. Anorectal function in Crohn's disease. Ann. Chir. Gynaecol. 81, 43–47 (1992).
Andersson, P., Olaison, G., Hallbook, O., Boeryd, B. & Sjodahl, R. Increased anal resting pressure and rectal sensitivity in Crohn's disease. Dis. Colon Rectum 46, 1685–1689 (2003).
Loening-Baucke, V., Metcalf, A. M. & Shirazi, S. Rectosigmoid motility in patients with quiescent and active ulcerative colitis. Am. J. Gastroenterol. 84, 34–39 (1989).
Bassotti, G. et al. Colonic propulsive and postprandial motor activity in patients with ulcerative colitis in remission. Eur. J. Gastroenterol. Hepatol. 18, 507–510 (2006).
Annese, V. et al. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand. J. Gastroenterol. 32, 1107–1117 (1997).
Minderhoud, I. M., Smout, A. J., Oldenburg, B. & Samsom, M. A pilot study on chemospecific duodenal visceral sensitivity in inflammatory bowel disease in remission. Digestion 73, 151–159 (2006).
Collins, S. M., Van Assche, G. & Hogaboam, C. Alterations in enteric nerve and smooth-muscle function in inflammatory bowel diseases. Inflamm. Bowel Dis. 3, 38–48 (1997).
Geboes, K. & Collins, S. Structural abnormalities of the nervous system in Crohn's disease and ulcerative colitis. Neurogastroenterol. Motil. 10, 189–202 (1998).
Belai, A., Boulos, P. B., Robson, T. & Burnstock, G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut 40, 767–774 (1997).
Schneider, J. et al. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn's disease. Neurogastroenterol. Motil. 13, 255–264 (2001).
Sjolund, K. et al. Peptide-containing nerve fibres in the gut wall in Crohn's disease. Gut 24, 724–733 (1983).
Todorovic, V. et al. Colonic vasoactive intestinal polypeptide (VIP) in ulcerative colitis—a radioimmunoassay and immunohistochemical study. Hepatogastroenterology 43, 483–488 (1996).
Lee, C. M., Kumar, R. K., Lubowski, D. Z. & Burcher, E. Neuropeptides and nerve growth in inflammatory bowel diseases: a quantitative immunohistochemical study. Dig. Dis. Sci. 47, 495–502 (2002).
Straub, R. H. et al. Anti-inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut 57, 911–921 (2008).
Dvorak, A. M., Osage, J. E., Monahan, R. A. & Dickersin, G. R. Crohn's disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum. Pathol. 11, 620–634 (1980).
Oehmichen, M. & Reifferscheid, P. Intramural ganglion cell degeneration in inflammatory bowel disease. Digestion 15, 482–496 (1977).
Roberts, P. J., Morgan, K., Miller, R., Hunter, J. O. & Middleton, S. J. Neuronal COX-2 expression in human myenteric plexus in active inflammatory bowel disease. Gut 48, 468–472 (2001).
Nadorra, R., Landing, B. H. & Wells, T. R. Intestinal plexuses in Crohn's disease and ulcerative colitis in children: pathologic and microdissection studies. Pediatr. Pathol. 6, 267–287 (1986).
Strobach, R. S., Ross, A. H., Markin, R. S., Zetterman, R. K. & Linder, J. Neural patterns in inflammatory bowel disease: an immunohistochemical survey. Mod. Pathol. 3, 488–493 (1990).
Neunlist, M. et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut 52, 84–90 (2003).
Gulbransen, B. D. et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 18, 600–604 (2012).
Bassotti, G. et al. Enteric neuroglial apoptosis in inflammatory bowel diseases. J. Crohns Colitis 3, 264–270 (2009).
Geboes, K. et al. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology 103, 439–447 (1992).
Koretz, K., Momburg, F., Otto, H. F. & Moller, P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn's disease. Am. J. Pathol. 129, 493–502 (1987).
Ohlsson, B., Sundkvist, G. & Lindgren, S. Subclinical sympathetic neuropathy appears early in the course of Crohn's disease. BMC Gastroenterol. 7, 33 (2007).
Ferrante, M. et al. The value of myenteric plexitis to predict early postoperative Crohn's disease recurrence. Gastroenterology 130, 1595–1606 (2006).
Sokol, H. et al. Plexitis as a predictive factor of early postoperative clinical recurrence in Crohn's disease. Gut 58, 1218–1225 (2009).
Rao, S. S., Read, N. W., Davison, P. A., Bannister, J. J. & Holdsworth, C. D. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology 93, 1270–1275 (1987).
Farthing, M. J. & Lennard-Jones, J. E. Sensibility of the rectum to distension and the anorectal distension reflex in ulcerative colitis. Gut 19, 64–69 (1978).
Drewes, A. M. et al. Pain and mechanical properties of the rectum in patients with active ulcerative colitis. Inflamm. Bowel Dis. 12, 294–303 (2006).
Buchmann, P., Kolb, E. & Alexander-Williams, J. Pathogenesis of urgency in defaecation in Crohn's disease. Digestion 22, 310–316 (1981).
Docherty, M. J., Jones, R. C. 3rd & Wallace, M. S. Managing pain in inflammatory bowel disease. Gastroenterol. Hepatol. (NY) 7, 592–601 (2011).
Schirbel, A. et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 16, 3168–3177 (2010).
Greenley, R. N., Kunz, J. H., Schurman, J. V. & Swanson, E. Abdominal pain and health related quality of life in pediatric inflammatory bowel disease. J. Pediatr. Psychol. 38, 63–71 (2013).
Wagtmans, M. J., Verspaget, H. W., Lamers, C. B. & van Hogezand, R. A. Crohn's disease in the elderly: a comparison with young adults. J. Clin. Gastroenterol. 27, 129–133 (1998).
Ibeakanma, C. & Vanner, S. TNFalpha is a key mediator of the pronociceptive effects of mucosal supernatant from human ulcerative colitis on colonic DRG neurons. Gut 59, 612–621 (2010).
Farrokhyar, F., Marshall, J. K., Easterbrook, B. & Irvine, E. J. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm. Bowel Dis. 12, 38–46 (2006).
Minderhoud, I. M., Oldenburg, B., Wismeijer, J. A., van Berge Henegouwen, G. P. & Smout, A. J. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig. Dis. Sci. 49, 469–474 (2004).
Loening-Baucke, V., Metcalf, A. M. & Shirazi, S. Anorectal manometry in active and quiescent ulcerative colitis. Am. J. Gastroenterol. 84, 892–897 (1989).
Mayer, E. A. et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 115, 398–409 (2005).
Furlan, R. et al. Sympathetic overactivity in active ulcerative colitis: effects of clonidine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R224–R232 (2006).
Ganguli, S. C. et al. A comparison of autonomic function in patients with inflammatory bowel disease and in healthy controls. Neurogastroenterol. Motil. 19, 961–967 (2007).
Straub, R. H. et al. Association of autonomic nervous hyperreflexia and systemic inflammation in patients with Crohn's disease and ulcerative colitis. J. Neuroimmunol. 80, 149–157 (1997).
Lechin, F. et al. Treatment of ulcerative colitis with clonidine. J. Clin. Pharmacol. 25, 219–226 (1985).
Mouzas, I. A. et al. Autonomic imbalance during the day in patients with inflammatory bowel disease in remission. Evidence from spectral analysis of heart rate variability over 24 hours. Dig. Liver Dis. 34, 775–780 (2002).
Sharma, P., Makharia, G. K., Ahuja, V., Dwivedi, S. N. & Deepak, K. K. Autonomic dysfunctions in patients with inflammatory bowel disease in clinical remission. Dig. Dis. Sci. 54, 853–861 (2009).
Lovell, R. M. & Ford, A. C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721 (2012).
Camilleri, M. Peripheral mechanisms in irritable bowel syndrome. N. Engl. J. Med. 367, 1626–1635 (2012).
Berman, S. et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage 63, 1854–1863 (2012).
Bercik, P., Verdu, E. F. & Collins, S. M. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol. Clin. North. Am 34, 235–245 (2005).
Spiller, R. C. Inflammation as a basis for functional GI disorders. Best Pract. Res. Clin. Gastroenterol. 18, 641–661 (2004).
Peters, G. A. & Bargen, J. A. The irritable bowel syndrome. Gastroenterology 3, 399–402 (1944).
Barratt, S. M. et al. Prodromal irritable bowel syndrome may be responsible for delays in diagnosis in patients presenting with unrecognized Crohn's disease and celiac disease, but not ulcerative colitis. Dig. Dis. Sci. 56, 3270–3275 (2011).
Burgmann, T. et al. The Manitoba Inflammatory Bowel Disease Cohort Study: prolonged symptoms before diagnosis—how much is irritable bowel syndrome? Clin. Gastroenterol. Hepatol. 4, 614–620 (2006).
Ansari, R. et al. Ulcerative colitis and irritable bowel syndrome: relationships with quality of life. Eur. J. Gastroenterol. Hepatol. 20, 46–50 (2008).
Olbe, L. Concept of Crohn's disease being conditioned by four main components, and irritable bowel syndrome being an incomplete Crohn's disease. Scand. J. Gastroenterol. 43, 234–241 (2008).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Bertiaux-Vandaele, N. et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 106, 2165–2173 (2011).
Dunlop, S. P. et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 101, 1288–1294 (2006).
Hughes, P. A. et al. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am. J. Gastroenterol. 108, 1066–1074 (2013).
Donovan, J. & Grundy, D. Endocannabinoid modulation of jejunal afferent responses to LPS. Neurogastroenterol. Motil. 24, 956–e465 (2012).
Ochoa-Cortes, F. et al. Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G723–G732 (2010).
Wang, B. et al. Lipopolysaccharide-induced changes in mesenteric afferent sensitivity of rat jejunum in vitro: role of prostaglandins. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G254–G260 (2005).
Liu, C. Y., Mueller, M. H., Rogler, G., Grundy, D. & Kreis, M. E. Differential afferent sensitivity to mucosal lipopolysaccharide from Salmonella typhimurium and Escherichia coli in the rat jejunum. Neurogastroenterol. Motil. 21, 1335–e129 (2009).
Barbara, G. et al. Postinfectious irritable bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 48 (Suppl. 2), S95–S97 (2009).
Spiller, R. & Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 136, 1979–1988 (2009).
Thabane, M. & Marshall, J. K. Post-infectious irritable bowel syndrome. World J. Gastroenterol. 15, 3591–3596 (2009).
Barbara, G., De Giorgio, R., Stanghellini, V., Cremon, C. & Corinaldesi, R. A role for inflammation in irritable bowel syndrome? Gut 51 (Suppl. 1), 41–44 (2002).
Barbara, G. et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702 (2004).
Hughes, P. A. et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 62, 1456–1465 (2013).
Liebregts, T. et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am. J. Gastroenterol. 106, 1089–1098 (2011).
Liebregts, T. et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 132, 913–920 (2007).
Ohman, L. et al. T-cell activation in patients with irritable bowel syndrome. Am. J. Gastroenterol. 104, 1205–1212 (2009).
Lindberg, G. et al. Full-thickness biopsy findings in chronic intestinal pseudo-obstruction and enteric dysmotility. Gut 58, 1084–1090 (2009).
Ohlsson, B., Ekblad, E., Veress, B., Montgomery, A. & Janciauskiene, S. Antibodies against gonadotropin-releasing hormone (GnRH) and destruction of enteric neurons in 3 patients suffering from gastrointestinal dysfunction. BMC Gastroenterol. 10, 48 (2010).
Gwee, K. A. et al. Increased rectal mucosal expression of interleukin 1β in recently acquired post-infectious irritable bowel syndrome. Gut 52, 523–526 (2003).
Chadwick, V. S. et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 122, 1778–1783 (2002).
Barbara, G. et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132, 26–37 (2007).
Buhner, S. et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137, 1425–1434 (2009).
Gonsalkorale, W. M., Perrey, C., Pravica, V., Whorwell, P. J. & Hutchinson, I. V. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut 52, 91–93 (2003).
Shea-Donohue, T., Notari, L., Sun, R. & Zhao, A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol. Motil. 24, 802–811 (2012).
Crosthwaite, A. I., Huizinga, J. D. & Fox, J. A. Jejunal circular muscle motility is decreased in nematode-infected rat. Gastroenterology 98, 59–65 (1990).
Grossi, L., McHugh, K. & Collins, S. M. On the specificity of altered muscle function in experimental colitis in rats. Gastroenterology 104, 1049–1056 (1993).
Jodal, M., Wingren, U., Jansson, M., Heidemann, M. & Lundgren, O. Nerve involvement in fluid transport in the inflamed rat jejunum. Gut 34, 1526–1530 (1993).
Vermillion, D. L. & Collins, S. M. Increased responsiveness of jejunal longitudinal muscle in Trichinella-infected rats. Am. J. Physiol. 254, G124–G129 (1988).
Hosseini, J. M., Goldhill, J. M., Bossone, C., Pineiro-Carrero, V. & Shea-Donohue, T. Progressive alterations in circular smooth muscle contractility in TNBS-induced colitis in rats. Neurogastroenterol. Motil. 11, 347–356 (1999).
Martinolle, J. P., Garcia-Villar, R., Fioramonti, J. & Bueno, L. Altered contractility of circular and longitudinal muscle in TNBS-inflamed guinea pig ileum. Am. J. Physiol. 272, G1258–G1267 (1997).
Moreels, T. G. et al. Effect of 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced ileitis on the motor function of non-inflamed rat gastric fundus. Neurogastroenterol. Motil. 13, 339–352 (2001).
Strong, D. S. et al. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J. Physiol. 588, 847–859 (2010).
Perdue, M. H., Marshall, J. & Masson, S. Ion transport abnormalities in inflamed rat jejunum. Involvement of mast cells and nerves. Gastroenterology 98, 561–567 (1990).
Masson, S. D. et al. Nippostrongylus brasiliensis infection evokes neuronal abnormalities and alterations in neurally regulated electrolyte transport in rat jejunum. Parasitology 113, 173–182 (1996).
Sanchez de Medina, F. et al. Disturbances of colonic ion secretion in inflammation: role of the enteric nervous system and cAMP. Pflugers Arch. 444, 378–388 (2002).
MacNaughton, W. K., Lowe, S. S. & Cushing, K. Role of nitric oxide in inflammation-induced suppression of secretion in a mouse model of acute colitis. Am. J. Physiol. 275, G1353–G1360 (1998).
Perez-Navarro, R., Ballester, I., Zarzuelo, A. & Sanchez de Medina, F. Disturbances in epithelial ionic secretion in different experimental models of colitis. Life Sci. 76, 1489–1501 (2005).
Motomura, Y. et al. Mechanisms underlying gut dysfunction in a murine model of chronic parasitic infection. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1354–G1360 (2010).
Bercik, P. et al. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology 127, 179–187 (2004).
Krauter, E. M. et al. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol. Motil. 19, 990–1000 (2007).
Lomax, A. E., O'Hara, J. R., Hyland, N. P., Mawe, G. M. & Sharkey, K. A. Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G482–G491 (2007).
Van Nassauw, L., Bogers, J., Van Marck, E. & Timmermans, J. P. Role of reactive nitrogen species in neuronal cell damage during intestinal schistosomiasis. Cell Tissue Res. 303, 329–336 (2001).
Demedts, I. et al. Neural mechanisms of early postinflammatory dysmotility in rat small intestine. Neurogastroenterol. Motil. 18, 1102–1111 (2006).
Bradley, J. S. Jr, Parr, E. J. & Sharkey, K. A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 289, 455–461 (1997).
Poli, E., Lazzaretti, M., Grandi, D., Pozzoli, C. & Coruzzi, G. Morphological and functional alterations of the myenteric plexus in rats with TNBS-induced colitis. Neurochem. Res. 26, 1085–1093 (2001).
Sarnelli, G. et al. Myenteric neuronal loss in rats with experimental colitis: role of tissue transglutaminase-induced apoptosis. Dig. Liver Dis. 41, 185–193 (2009).
Linden, D. R. et al. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol. Motil. 17, 751–760 (2005).
Boyer, L. et al. Myenteric plexus injury and apoptosis in experimental colitis. Auton. Neurosci. 117, 41–53 (2005).
Linden, D. R. Colitis is associated with a loss of intestinofugal neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1096–G1104 (2012).
Margolis, K. G. et al. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology 141, 588–598 (2011).
Mya, G. H., Ng, W. F., Cheng, W. & Saing, H. Colonic hyperganglionosis presenting as neonatal enterocolitis and multiple strictures. J. Pediatr. Surg. 29, 1628–1630 (1994).
Linden, D. R., Sharkey, K. A. & Mawe, G. M. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J. Physiol. 547, 589–601 (2003).
Chen, Z. et al. Cyclic AMP signaling contributes to neural plasticity and hyperexcitability in AH sensory neurons following intestinal Trichinella spiralis-induced inflammation. Int. J. Parasitol. 37, 743–761 (2007).
Linden, D. R., Sharkey, K. A., Ho, W. & Mawe, G. M. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J. Physiol. 557, 191–205 (2004).
Krauter, E. M., Linden, D. R., Sharkey, K. A. & Mawe, G. M. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation-induced synaptic facilitation. J. Physiol. 581, 787–800 (2007).
Lomax, A. E., Mawe, G. M. & Sharkey, K. A. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J. Physiol. 564, 863–875 (2005).
Blandizzi, C. et al. Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by α2-adrenoceptors in the presence of experimental colitis. Br. J. Pharmacol. 139, 309–320 (2003).
Collins, S. M., Blennerhassett, P. A., Blennerhassett, M. G. & Vermillion, D. L. Impaired acetylcholine release from the myenteric plexus of Trichinella-infected rats. Am. J. Physiol. 257, G898–G903 (1989).
Hoffman, J. M., McKnight, N. D., Sharkey, K. A. & Mawe, G. M. The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol. Motil. 23, 673–e279 (2011).
Nurgali, K. et al. Morphological and functional changes in guinea-pig neurons projecting to the ileal mucosa at early stages after inflammatory damage. J. Physiol. 589, 325–339 (2011).
O'Hara, J. R., Lomax, A. E., Mawe, G. M. & Sharkey, K. A. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut 56, 186–194 (2007).
Hons, I. M., Burda, J. E., Grider, J. R., Mawe, G. M. & Sharkey, K. A. Alterations to enteric neural signaling underlie secretory abnormalities of the ileum in experimental colitis in the guinea pig. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G717–G726 (2009).
Linden, D. R. Enhanced excitability of guinea pig ileum myenteric AH neurons during and following recovery from chemical colitis. Neurosci. Lett. 545, 91–95 (2013).
Jacobson, K., McHugh, K. & Collins, S. M. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology 112, 156–162 (1997).
Motagally, M. A., Neshat, S. & Lomax, A. E. Inhibition of sympathetic N-type voltage-gated Ca2+ current underlies the reduction in norepinephrine release during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1077–G1084 (2009).
Birch, D., Knight, G. E., Boulos, P. B. & Burnstock, G. Analysis of innervation of human mesenteric vessels in non-inflamed and inflamed bowel—a confocal and functional study. Neurogastroenterol. Motil. 20, 660–670 (2008).
Lomax, A. E., O'Reilly, M., Neshat, S. & Vanner, S. J. Sympathetic vasoconstrictor regulation of mouse colonic submucosal arterioles is altered in experimental colitis. J. Physiol. 583, 719–730 (2007).
Mathison, R. & Davison, J. S. Capsaicin sensitive nerves in the jejunum of Nippostrongylus brasiliensis-sensitized rats participate in a cardiovascular depressor reflex. Naunyn Schmiedebergs Arch. Pharmacol. 348, 638–642 (1993).
Mathison, R. & Davison, J. S. Regulation of jejunal arterioles by capsaicin-sensitive nerves in Nippostrongylus brasiliensis-sensitized rats. J. Pharmacol. Exp. Ther. 273, 337–343 (1995).
Neshat, S. et al. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G399–G405 (2009).
Linden, D. R. Enhanced excitability of guinea pig inferior mesenteric ganglion neurons during and following recovery from chemical colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1067–G1075 (2012).
Dong, X. X., Thacker, M., Pontell, L., Furness, J. B. & Nurgali, K. Effects of intestinal inflammation on specific subgroups of guinea-pig celiac ganglion neurons. Neurosci. Lett. 444, 231–235 (2008).
Castex, N. et al. Brain Fos expression and intestinal motor alterations during nematode-induced inflammation in the rat. Am. J. Physiol. 274, G210–G216 (1998).
Holzer, P. et al. Increase in gastric acid-induced afferent input to the brainstem in mice with gastritis. Neuroscience 145, 1108–1119 (2007).
Wultsch, T. et al. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach–brainstem axis. Pain 134, 245–253 (2008).
Ghia, J. E., Blennerhassett, P. & Collins, S. M. Vagus nerve integrity and experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G560–G567 (2007).
Ghia, J. E., Blennerhassett, P., Kumar-Ondiveeran, H., Verdu, E. F. & Collins, S. M. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130 (2006).
Matteoli, G. & Boeckxstaens, G. E. The vagal innervation of the gut and immune homeostasis. Gut 62, 1214–1222 (2013).
Page, A. J., O'Donnell, T. A. & Blackshaw, L. A. P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J. Physiol. 523, 403–411 (2000).
Yu, S. & Ouyang, A. Effect of synthetic cationic protein on mechanoexcitability of vagal afferent nerve subtypes in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G1052–G1058 (2011).
Yu, S., Gao, G., Peterson, B. Z. & Ouyang, A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G34–G42 (2009).
Kang, Y. M., Bielefeldt, K. & Gebhart, G. F. Sensitization of mechanosensitive gastric vagal afferent fibers in the rat by thermal and chemical stimuli and gastric ulcers. J. Neurophysiol. 91, 1981–1989 (2004).
Hughes, P. A. et al. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58, 1333–1341 (2009).
De Schepper, H. U. et al. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J. Physiol. 586, 5247–5258 (2008).
Castro, J. et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-c and extracellular cyclic guanosine 3′, 5′-monophosphate. Gastroenterology 145, 1334–1346 (2013).
Jones, R. C. 3rd, Xu, L. & Gebhart, G. F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J. Neurosci. 25, 10981–10989 (2005).
Brierley, S. M. et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137, 2084–2095 (2009).
Cattaruzza, F. et al. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G81–G91 (2010).
Brierley, S. M. et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134, 2059–2069 (2008).
Sipe, W. E. et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1288–G1298 (2008).
Jones, R. C. 3rd et al. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133, 184–194 (2007).
Miranda, A. et al. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 148, 1021–1032 (2007).
Brierley, S. M., Jones, R. C. 3rd, Xu, L., Gebhart, G. F. & Blackshaw, L. A. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol. Motil. 17, 854–862 (2005).
Coldwell, J. R., Phillis, B. D., Sutherland, K., Howarth, G. S. & Blackshaw, L. A. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J. Physiol. 579, 203–213 (2007).
Cenac, N. et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 59, 481–488 (2010).
Cattaruzza, F. et al. Cathepsin S is activated during colitis and causes visceral hyperalgesia by a PAR2-dependent mechanism in mice. Gastroenterology 141, 1864–1874 (2011).
Engel, M. A. et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 141, 1346–1358 (2011).
Vergnolle, N. et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br. J. Pharmacol. 159, 1161–1173 (2010).
D'Aldebert, E. et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 140, 275–285 (2011).
Brierley, S. M. et al. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J. Physiol. 567, 267–281 (2005).
Feng, B. et al. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G676–G683 (2012).
Bielefeldt, K., Ozaki, N. & Gebhart, G. F. Experimental ulcers alter voltage-sensitive sodium currents in rat gastric sensory neurons. Gastroenterology 122, 394–405 (2002).
Dang, K., Bielefeldt, K. & Gebhart, G. F. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G573–G579 (2004).
Hillsley, K. et al. Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice. J. Physiol. 576, 257–267 (2006).
Stewart, T., Beyak, M. J. & Vanner, S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J. Physiol. 552, 797–807 (2003).
Beyak, M. J., Ramji, N., Krol, K. M., Kawaja, M. D. & Vanner, S. J. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G845–G855 (2004).
Ibeakanma, C. et al. Citrobacter rodentium colitis evokes post-infectious hyperexcitability of mouse nociceptive colonic dorsal root ganglion neurons. J. Physiol. 587, 3505–3521 (2009).
King, D. E., Macleod, R. J. & Vanner, S. J. Trinitrobenzenesulphonic acid colitis alters Na 1.8 channel expression in mouse dorsal root ganglia neurons. Neurogastroenterol. Motil. 21, 880–e64 (2009).
Ozaki, N., Bielefeldt, K., Sengupta, J. N. & Gebhart, G. F. Models of gastric hyperalgesia in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G666–G676 (2002).
Traub, R. J. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11, 2113–2116 (2000).
Traub, R. J. & Murphy, A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain 95, 93–102 (2002).
Aerssens, J. et al. Alterations in the brain-gut axis underlying visceral chemosensitivity in Nippostrongylus brasiliensis-infected mice. Gastroenterology 132, 1375–1387 (2007).
Rong, W., Keating, C., Sun, B., Dong, L. & Grundy, D. Purinergic contribution to small intestinal afferent hypersensitivity in a murine model of postinfectious bowel disease. Neurogastroenterol. Motil. 21, 665–671 (2009).
Keating, C. et al. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J. Physiol. 586, 4517–4530 (2008).
Keating, C., Pelegrin, P., Martinez, C. M. & Grundy, D. P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J. Immunol. 187, 1467–1474 (2011).
Traub, R. J. et al. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology 135, 2075–2083 (2008).
Adam, B. et al. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain 123, 179–186 (2006).
Marshall, J. K. et al. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut 59, 605–611 (2010).
Gschossmann, J. M. et al. Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig. Dis. Sci. 49, 96–101 (2004).
Lamb, K., Zhong, F., Gebhart, G. F. & Bielefeldt, K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G451–G457 (2006).
Harrington, A. M. et al. Sprouting of colonic afferent central terminals and increased spinal mitogen-activated protein kinase expression in a mouse model of chronic visceral hypersensitivity. J. Comp. Neurol. 520, 2241–2255 (2012).
Hughes, P. A. et al. Increased kappa-opioid receptor expression and function during chronic visceral hypersensitivity. Gut http://dx.doi.org/10.1136/gutjnl-2013-306240.
de Araujo, A. D. et al. Selenoether oxytocin analogues have analgesic properties in a mouse model of chronic abdominal pain. Nat. Commun. 5, 3165 (2014).
Larsson, M. H., Rapp, L. & Lindstrom, E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol. Motil. 18, 144–152 (2006).
Verma-Gandhu, M. et al. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut 56, 358–364 (2007).
Valdez-Morales, E. et al. Release of endogenous opioids during a chronic IBD model suppresses the excitability of colonic DRG neurons. Neurogastroenterol. Motil. 25, 39–46 (2013).
Eijkelkamp, N. et al. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G749–G757 (2007).
Matsumoto, K. et al. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab. Invest. 92, 769–782 (2012).
Shinoda, M., La, J. H., Bielefeldt, K. & Gebhart, G. F. Altered purinergic signaling in colorectal dorsal root ganglion neurons contributes to colorectal hypersensitivity. J. Neurophysiol. 104, 3113–3123 (2010).
Ibeakanma, C. et al. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology 141, 2098–2108 (2011).
Liebregts, T. et al. Psychological stress and the severity of post-inflammatory visceral hyperalgesia. Eur. J. Pain 11, 216–222 (2007).
Yang, C. Q., Wei, Y. Y., Zhong, C. J. & Duan, L. P. Essential role of mast cells in the visceral hyperalgesia induced by T. spiralis infection and stress in rats. Mediators Inflamm. 2012, 294070 (2012).
Yang, C. Q. et al. Vesicular glutamate transporter-3 contributes to visceral hyperalgesia induced by Trichinella spiralis infection in rats. Dig. Dis. Sci. 57, 865–872 (2012).
Larsson, M. H., Miketa, A. & Martinez, V. Lack of interaction between psychological stress and DSS-induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress 12, 434–444 (2009).
Winston, J., Shenoy, M., Medley, D., Naniwadekar, A. & Pasricha, P. J. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 132, 615–627 (2007).
Christianson, J. A., Bielefeldt, K., Malin, S. A. & Davis, B. M. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain 151, 540–549 (2010).
Xu, G. Y., Shenoy, M., Winston, J. H., Mittal, S. & Pasricha, P. J. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 57, 1230–1237 (2008).
Liu, L. S. et al. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterology 134, 2070–2079 (2008).
Liu, L. S., Shenoy, M. & Pasricha, P. J. The analgesic effects of the GABAB receptor agonist, baclofen, in a rodent model of functional dyspepsia. Neurogastroenterol. Motil. 23, 356–361 (2011).
Winston, J. H. & Sarna, S. K. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology 144, 570–579 (2013).
Larson, S. J., Collins, S. M. & Weingarten, H. P. Dissociation of temperature changes and anorexia after experimental colitis and LPS administration in rats. Am. J. Physiol. 271, R967–R972 (1996).
McHugh, K. J., Weingarten, H. P., Keenan, C., Wallace, J. L. & Collins, S. M. On the suppression of food intake in experimental models of colitis in the rat. Am. J. Physiol. 264, R871–R876 (1993).
Lyte, M., Li, W., Opitz, N., Gaykema, R. P. & Goehler, L. E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 89, 350–357 (2006).
Bercik, P. et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139, 2102–2112 (2010).
Gareau, M. G. et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317 (2011).
Bercik, P. et al. Role of gut–brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R587–R594 (2009).
McHugh, K. J., Collins, S. M. & Weingarten, H. P. Central interleukin-1 receptors contribute to suppression of feeding after acute colitis in the rat. Am. J. Physiol. 266, R1659–R1663 (1994).
De Schepper, H. U. et al. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G245–G253 (2008).
Hong, S. et al. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 58, 202–210 (2009).
Karanjia, R., Spreadbury, I., Bautista-Cruz, F., Tsang, M. E. & Vanner, S. Activation of protease-activated receptor-4 inhibits the intrinsic excitability of colonic dorsal root ganglia neurons. Neurogastroenterol. Motil. 21, 1218–1221 (2009).
Harrington, A. M. et al. A novel role for TRPM8 in visceral afferent function. Pain 152, 1459–1468 (2011).
Acknowledgements
The authors wish to thank J. Applequist at the Mayo Clinic College of Medicine and J. Maddern at the University of Adelaide, Australia, for their secretarial assistance. S.M.B. is supported by an NHMRC R.D. Wright Biomedical Fellowship and by NHMRC Australia Project grants (1008100, 1049682, 1049928 and 1063803). D.R.L. is supported by NIH grant DK76665.
Author information
Authors and Affiliations
Contributions
S.M.B. and D.R.L. contributed equally to the discussion of content, writing and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.M.B. receives research support from Ironwood Pharmaceuticals Inc. None of the details relating to this research support are discussed in this review. D.R.L. declares no competing interests.
Supplementary information
Supplementary Box 1
Other clinical gastrointestinal inflammatory disorders causing gut dysfunction (PDF 447 kb)
Supplementary Box 2
Non-neuronal influences on gastrointestinal function (PDF 287 kb)
Supplementary Table 1
Animal models of gastrointestinal infection/inflammation and the neuroplasticity and altered gut function they induce (PDF 198 kb)
Rights and permissions
About this article
Cite this article
Brierley, S., Linden, D. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 11, 611–627 (2014). https://doi.org/10.1038/nrgastro.2014.103
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.103
This article is cited by
-
A comparison of interferential current efficacy in elderly intervertebral disc degeneration patients with or without sarcopenia: a retrospective study
BMC Musculoskeletal Disorders (2024)
-
Dialogue between specialized gut cells and nerves contributes to sex bias of gut pain
Nature (2023)
-
Piezo2 regulates colonic mechanical sensitivity in a sex specific manner in mice
Nature Communications (2023)
-
Targeting the endocannabinoid system for the treatment of abdominal pain in irritable bowel syndrome
Nature Reviews Gastroenterology & Hepatology (2023)
-
Neuroimmunologie der allergischen Rhinitis
HNO (2023)