Key Points
-
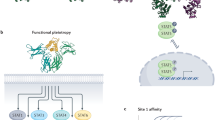
Type II cytokine receptors consist of 1 soluble and 11 transmembrane proteins that have 20–30% amino-acid identity in their extracellular domain.
-
The ligands have a common structure with six α-helices, organized as monomers or homodimers, and have 20–30% amino-acid identity.
-
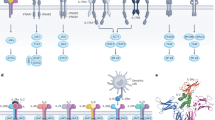
Receptors associate as heterodimers including common chains and a high level of complexity in cytokine–receptor interactions results from the fact that one particular cytokine can bind to two different receptor complexes, and that one particular receptor complex can bind several cytokines.
-
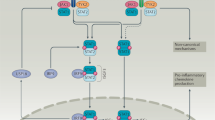
Type I interferons (IFNs), IL-28 and IL-29 have important roles in antiviral responses through the activation of signal transducer and activator of transcription 2 (STAT2).
-
Inflammatory responses are modulated by members of this family; IL-10 and IFN-γ have anti- and pro-inflammatory activities, respectively; IL-22 induces the production of acute-phase reactants and IL-20 regulates the proliferation of keratinocytes.
-
Antitumour activities have been described for type I IFNs and IL-24 delivered by adenoviruses through an unknown mechanism, potentially independent from IL-24 receptor activation.
Abstract
Class II cytokine receptors were originally defined on the basis of sequence homologies in the extracellular domains of receptors for interferons (IFNs) and interleukin-10 (IL-10), and the ligands, known as class II cytokines, also have a common structure. More recently, a series of new receptors and cytokines that belong to this family have been discovered. The therapeutic potential of the 'old' members of this family, IFNs and IL-10, is recognized in the clinic, and the existence of structurally related molecules is raising expectations for additional clinical applications. In this review, I discuss both structural and biological data that are emerging about this family of receptors and ligands, to highlight the potential applications of modulating the activity of these cytokines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Isaacs, A. & Lindenmann, J. Virus interference: 1. The interferon. Proc. R. Soc. Lond. B 147, 258–267 (1957).
Goodbourn, S., Didcock, L. & Randall, R. E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81, 2341–2364 (2000).
Dalton, D. K. et al. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 (1993).
Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Kotenko, S. V. & Pestka, S. Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene 19, 2557–2565 (2000).
Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Walter, M. R. Crystal structures of α-helical cytokine-receptor complexes: we've only scratched the surface. Biotechniques Suppl, S50–S57 (2002).
Kotenko, S. V. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 13, 223–240 (2002).
Dumoutier, L. & Renauld, J. C. Viral and cellular interleukin-10 (IL-10)-related cytokines: from structures to functions. Eur. Cytokine Netw. 13, 5–15 (2002).
Fickenscher, H. et al. The interleukin-10 family of cytokines. Trends Immunol. 23, 89–96 (2002).
Jiang, H., Lin, J. J., Su, Z. Z., Goldstein, N. I. & Fisher, P. B. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 11, 2477–2486 (1995).
Knappe, A., Hor, S., Wittmann, S. & Fickenscher, H. Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J. Virol. 74, 3881–3887 (2000).
Dumoutier, L., Louahed, J. & Renauld, J. C. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 164, 1814–1819 (2000).
Gallagher, G. et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10). Genes Immun. 1, 442–450 (2000).
Blumberg, H. et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell 104, 9–19 (2001). This study showed that overexpression of interleukin-20 (IL-20) in transgenic mice results in abnormal differentiation and proliferation of keratinocytes — a phenotype reminiscent of psoriatic skin in humans.
Sheppard, P. et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nature Immunol. 4, 63–68 (2003). This paper described the cloning of IL-28A, IL-28B, IL-29 and their receptor, and shows that they exert antiviral activity. Similar observations were reported by Kotenko et al . in reference 17.
Kotenko, S. V. et al. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nature Immunol. 4, 69–77 (2003).
Oritani, K. et al. Limitin: An interferon-like cytokine that preferentially influences B-lymphocyte precursors. Nature Med. 6, 659–666 (2000).
Chang, C. et al. Crystal structure of interleukin-19 defines a new subfamily of helical cytokines. J. Biol. Chem. 278, 3308–3313 (2003).
Nagem, R. A. et al. Crystal structure of recombinant human interleukin-22. Structure (Camb) 10, 1051–1062 (2002).
Karpusas, M. et al. The crystal structure of human interferon β at 2. 2-A resolution. Proc. Natl Acad. Sci. USA 94, 11813–11818 (1997).
Zdanov, A. et al. Crystal structure of interleukin-10 reveals the functional dimer with an unexpected topological similarity to interferon γ. Structure 3, 591–601 (1995).
Walter, M. R. & Nagabhushan, T. L. Crystal structure of interleukin 10 reveals an interferon γ-like fold. Biochemistry 34, 12118–12125 (1995).
Bazan, J. F. Shared architecture of hormone binding domains in type I and II interferon receptors. Cell 61, 753–754 (1990).
Josephson, K., Logsdon, N. J. & Walter, M. R. Crystal structure of the IL-10/IL-10R1 complex reveals a shared receptor binding site. Immunity 15, 35–46 (2001). This study described the crystal structure of IL-10 bound to a soluble form of IL-10 receptor 1 (sIL-10R1), showing that several residues in the IL-10–sIL-10R1 interface are conserved in all IL-10 homologues and their receptors.
Kotenko, S. V. et al. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J 16, 5894–5903 (1997).
Kotenko, S. V. et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rβ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 276, 2725–2732 (2001).
Dumoutier, L., Van Roost, E., Colau, D. & Renauld, J. C. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl Acad. Sci. USA 97, 10144–10149 (2000). This study reported the cloning of human IL-22 and showed its activity as an inducer of the acute-phase response through the IL-10Rβ chain.
Xie, M. H. et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 275, 31335–31339 (2000).
Lewerenz, M., Mogensen, K. E. & Uze, G. Shared receptor components but distinct complexes for α and β interferons. J. Mol. Biol. 282, 585–599 (1998).
Riewald, M. & Ruf, W. Orchestration of coagulation protease signaling by tissue factor. Trends Cardiovasc. Med. 12, 149–154 (2002).
Dumoutier, L., Lejeune, D., Colau, D. & Renauld, J. C. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166, 7090–7095 (2001).
Kotenko, S. V. et al. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J. Immunol. 166, 7096–7103 (2001).
Xu, W. et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc. Natl Acad. Sci. USA 98, 9511–9516 (2001).
Gruenberg, B. H. et al. A novel, soluble homologue of the human IL-10 receptor with preferential expression in placenta. Genes Immun. 2, 329–334 (2001).
Mantovani, A., Locati, M., Vecchi, A., Sozzani, S. & Allavena, P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 22, 328–336 (2001).
Lejeune, D. et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 277, 33676–33682 (2002).
Decker, T., Stockinger, S., Karaghiosoff, M., Muller, M. & Kovarik, P. IFNs and STATs in innate immunity to microorganisms. J. Clin. Invest. 109, 1271–1277 (2002).
Muller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Dumoutier, L., Lejeune, D., Hor, S., Fickenscher, H. & Renauld, J. C. Cloning of a new type II cytokine receptor activating signal transducer and activator of transcription (STAT)1, STAT2 and STAT3. Biochem. J. 370, 391–396 (2003).
Schijns, V. E., Wierda, C. M., van Hoeij, M. & Horzinek, M. C. Exacerbated viral hepatitis in IFN-γ receptor-deficient mice is not suppressed by IL-12. J. Immunol. 157, 815–821 (1996).
Jouanguy, E. et al. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11, 346–351 (1999).
Katze, M. G., He, Y. & Gale, M., Jr. Viruses and interferon: a fight for supremacy. Nature Rev. Immunol. 2, 675–687 (2002).
Alcami, A. & Smith, G. L. Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol. Today 16, 474–478 (1995).
Smith, G. L., Symons, J. A. & Alcami, A. Immune modulation by proteins secreted from cells infected by vaccinia virus. Arch. Virol. 15, 111–129 (1999).
Symons, J. A., Alcami, A. & Smith, G. L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81, 551–560 (1995).
Colamonici, O. R., Domanski, P., Sweitzer, S. M., Larner, A. & Buller, R. M. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon α transmembrane signaling. J. Biol. Chem. 270, 15974–15978 (1995).
Moore, K. W. et al. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein–Barr virus gene BCRFI. Science 248, 1230–1234 (1990).
Hsu, D. H. et al. Expression of interleukin-10 activity by Epstein–Barr virus protein BCRF1. Science 250, 830–832 (1990). This study showed that the Epstein–Barr virus BCRF1 protein mimics the activity of IL-10, indicating that BCRF1 might have a role in the interaction of the virus with the host's immune system.
Liu, Y. et al. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. J. Immunol. 158, 604–613 (1997).
Rode, H. J. et al. The genome of equine herpesvirus type 2 harbors an interleukin 10 (IL10)-like gene. Virus Genes 7, 111–116 (1993).
Fleming, S. B., McCaughan, C. A., Andrews, A. E., Nash, A. D. & Mercer, A. A. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J. Virol. 71, 4857–4861 (1997).
Kotenko, S. V., Saccani, S., Izotova, L. S., Mirochnitchenko, O. V. & Pestka, S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl Acad. Sci. USA 97, 1695–1700 (2000).
Lockridge, K. M. et al. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268, 272–280 (2000).
Spencer, J. V. et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76, 1285–1292 (2002).
Lee, H. J., Essani, K. & Smith, G. L. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology 281, 170–192 (2001).
Burdin, N., Peronne, C., Banchereau, J. & Rousset, F. Epstein–Barr virus transformation induces B lymphocytes to produce human interleukin 10. J. Exp. Med. 177, 295–304 (1993).
Howard, M., Muchamuel, T., Andrade, S. & Menon, S. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 177, 1205–1208 (1993).
Gerard, C. et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J. Exp. Med. 177, 547–550 (1993).
Ishida, H., Hastings, R., Thompson-Snipes, L. & Howard, M. Modified immunological status of anti-IL-10 treated mice. Cell. Immunol. 148, 371–384 (1993).
Heremans, H., Van Damme, J., Dillen, C., Dijkmans, R. & Billiau, A. Interferon-γ, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J. Exp. Med. 171, 1853–1869 (1990).
Rennick, D. M., Fort, M. M. & Davidson, N. J. Studies with IL-10−/− mice: an overview. J. Leukoc. Biol. 61, 389–396 (1997).
Hall, G. L., Compston, A. & Scolding, N. J. β-interferon and multiple sclerosis. Trends Neurosci. 20, 63–67 (1997).
Romagnani, S. The TH1/TH2 paradigm. Immunol. Today 18, 263–266 (1997).
Renauld, J. C. New insights into the role of cytokines in asthma. J. Clin. Pathol. 54, 577–589 (2001).
Fiorentino, D. F., Bond, M. W. & Mosmann, T. R. Two types of mouse T helper cell. IV. TH2 clones secrete a factor that inhibits cytokine production by TH1 clones. J. Exp. Med. 170, 2081–2095 (1989).
Fiorentino, D. F. et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by TH1 cells. J. Immunol. 146, 3444–3451 (1991).
Maloy, K. J. & Powrie, F. Regulatory T cells in the control of immune pathology. Nature Immunol. 2, 816–822 (2001).
Maloy, K. J. et al. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197, 111–119 (2003).
Wolk, K., Kunz, S., Asadullah, K. & Sabat, R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J. Immunol. 168, 5397–5402 (2002).
Schaefer, G., Venkataraman, C. & Schindler, U. Cutting edge: FISP (IL-4-induced secreted protein), a novel cytokine-like molecule secreted by TH2 cells. J. Immunol. 166, 5859–5863 (2001).
Liao, Y. C. et al. IL-19 induces production of IL-6 and TNF-α and results in cell apoptosis through TNF-α. J. Immunol. 169, 4288–4297 (2002).
Caudell, E. G. et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J. Immunol. 168, 6041–6046 (2002).
Dinarello, C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N. Engl. J. Med. 311, 1413–1418 (1984).
Heinrich, P. C., Castell, J. V. & Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 265, 621–636 (1990).
Aggarwal, S., Xie, M. H., Maruoka, M., Foster, J. & Gurney, A. L. Acinar cells of the pancreas are a target of interleukin-22. J. Interferon Cytokine Res. 21, 1047–1053 (2001).
Wang, M., Tan, Z., Zhang, R., Kotenko, S. V. & Liang, P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J. Biol. Chem. 277, 7341–7347 (2002).
Parrish-Novak, J. et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J. Biol. Chem. 277, 47517–47523 (2002).
Dumoutier, L., Leemans, C., Lejeune, D., Kotenko, S. V. & Renauld, J. C. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J. Immunol. 167, 3545–3549 (2001). This study showed that IL-19, IL-20 and IL-24, also known as melanocyte differentiation antigen 7 (MDA7), share similar receptor complexes, indicating that they should have overlapping activities.
Kirkwood, J. Cancer immunotherapy: the interferon-α experience. Semin. Oncol. 29, 18–26 (2002).
Ellerhorst, J. A. et al. Loss of MDA-7 expression with progression of melanoma. J. Clin. Oncol. 20, 1069–1074 (2002).
Ekmekcioglu, S. et al. Downregulated melanoma differentiation associated gene (mda-7) expression in human melanomas. Int. J. Cancer 94, 54–59 (2001).
Lebedeva, I. V. et al. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene 21, 708–718 (2002).
Sauane, M. et al. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 14, 35–51 (2003).
Su, Z. Z. et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc. Natl Acad. Sci. USA 95, 14400–14405 (1998).
Zhan, Q. et al. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 14, 2361–2371 (1994).
Sarkar, D. et al. mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc. Natl Acad. Sci. USA 99, 10054–10059 (2002).
Su, Z. Z. et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene 22, 1164–1180 (2003).
Pataer, A. et al. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via upregulation of the double-stranded RNA-dependent protein kinase (PKR). Cancer Res. 62, 2239–2243 (2002).
Huang, E. Y. et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene 20, 7051–7063 (2001).
Author information
Authors and Affiliations
Related links
Glossary
- CDNA SUBTRACTION CLONING
-
A method of cDNA cloning aimed to identify genes that are expressed specifically in a given tissue or after a particular stimulation.
- FIBRONECTIN DOMAINS
-
Fibronectin is an extracellular multiadhesive protein that binds to other matrix components, fibrin and cell-surface receptors of the integrin family. Fibronectin is composed of three types of repeating amino-acid sequences. The type III fibronectin domain is a 100 amino acid repeated domain that is involved in the binding of integrins.
- EPSTEIN–BARR VIRUS
-
(EBV). A double-stranded DNA virus of the herpes-virus family, which is the aetiologic agent of infectious mononucleosis and is associated with some B-cell malignant tumours and nasopharyngeal carcinoma. EBV infects B cells and some epithelial cells by specifically binding to complement receptor 2 (CD21).
- ACUTE-PHASE REACTANTS
-
(APRs). APRs consist of plasma proteins the expression of which is up- or downregulated during inflammation as the result of the endocrine activity of cytokines. Most APRs are produced by the liver and include components of the complement pathways, factors of the coagulation system, proteinase inhibitors, metal-binding proteins and other proteins involved in various infection-associated functions.
- PSORIASIS
-
A chronic skin disease that affects 1–2% of the population, in which the skin becomes inflamed, producing red, thickened areas with silvery scales, most often on the scalp, elbows, knees and lower back. Recent evidence points to a T-cell-mediated pathogenesis in genetically susceptible individuals, resulting in inflammation and epidermal hyperplasia.
- RNA-DEPENDENT PROTEIN KINASE
-
(PKR). PKR is a protein kinase that requires double-stranded RNA to exert its activity. This is supplied by virus RNA, which frequently loops back on itself to form double-stranded regions. One of the substrates of PKR is translation initiation factor 2 (IF2) — a factor involved in protein synthesis. IF2 is essential for the initiation complex of protein synthesis, but loses its activity after phosphorylation.
Rights and permissions
About this article
Cite this article
Renauld, JC. Class II cytokine receptors and their ligands: Key antiviral and inflammatory modulators. Nat Rev Immunol 3, 667–676 (2003). https://doi.org/10.1038/nri1153
Issue Date:
DOI: https://doi.org/10.1038/nri1153
This article is cited by
-
A transcriptomic analysis of skeletal muscle tissues reveals promising candidate genes and pathways accountable for different daily weight gain in Hanwoo cattle
Scientific Reports (2024)
-
RNA-Seq exploration of the influence of stress on meat quality in Spanish goats
Scientific Reports (2022)
-
G-protein coupled receptor, PI3K and Rho signaling pathways regulate the cascades of Tau and amyloid-β in Alzheimer’s disease
Molecular Biomedicine (2021)
-
Tofacitinib downregulates antiviral immune defence in keratinocytes and reduces T cell activation
Arthritis Research & Therapy (2021)
-
Role of intracellular signaling pathways and their inhibitors in the treatment of inflammation
Inflammopharmacology (2021)