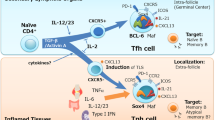
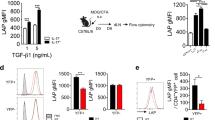
Abstract
CD4+ T helper 1 (TH1) and TH2 cells have long been regarded as two sides of a coin in terms of adaptive immune responses. However, as I discuss here, this concept needs to be reconsidered. In particular, recent data indicate that interleukin-17 (IL-17) is produced by TH cells that are distinct from the traditional TH1- and TH2-cell subsets. Furthermore, the generation of these IL-17-producing CD4+ T cells from naive precursors during immune responses is not dependent on the cytokines and transcription factors that mediate TH1- and TH2-cell development. Given that IL-17 has crucial roles in regulating tissue inflammation and the development of disease in several animal models of autoimmunity, I propose that IL-17-producing CD4+ T cells represent a distinct inflammatory TH-cell lineage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mosmann, T. R. & Coffman, R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Szabo, S. J., Sullivan, B. M., Peng, S. L. & Glimcher, L. H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 21, 713–758 (2003).
Glimcher, L. H. & Murphy, K. M. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14, 1693–1711 (2000).
Trinchieri, G., Pflanz, S. & Kastelein, R. A. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19, 641–644 (2003).
Zheng, W. & Flavell, R. A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Ho, I. C., Hodge, M. R., Rooney, J. W. & Glimcher, L. H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell 85, 973–983 (1996).
Chu, C.-Q., Wittmer, S. & Dalton, D. K. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192, 123–128 (2000).
Manoury-Schwartz, B. et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-γ receptors. J. Immunol. 158, 5501–5506 (1997).
Vermeire, K. et al. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J. Immunol. 158, 5507–5513 (1997).
Trembleau, S. et al. Pancreas-infiltrating Th1 cells and diabetes develop in IL-12-deficient nonobese diabetic mice. J. Immunol. 163, 2960–2968 (1999).
Hultgren, B., Huang, X., Dybdal, N. & Stewart, T. A. Genetic absence of γ-interferon delays but does not prevent diabetes in NOD mice. Diabetes 45, 812–817 (1996).
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Murphy, C. A. et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Vinuesa, C. G., Tangye, S. G., Moser, B. & Mackay, C. R. Follicular B helper T cells in antibody responses and autoimmunity. Nature Rev. Immunol. 5, 853–865 (2005).
Aggarwal, S. & Gurney, A. L. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 71, 1–8 (2002).
Moseley, T. A., Haudenschild, D. R., Rose, L. & Reddi, A. H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 (2003).
Kolls, J. K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Schwandner, R., Yamaguchi, K. & Cao, Z. Requirement of tumour necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 191, 1233–1240 (2000).
Ruddy, M. J. et al. Functional cooperation between interleukin-17 and tumour necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279, 2559–2567 (2004).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 6, 1133–1141 (2005).
Shen, F., Ruddy, M. J., Plamondon, P. & Gaffen, S. L. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-α-induced genes in bone cells. J. Leukoc. Biol. 77, 388–399 (2005).
Ye, P. et al. Requirement of interleukin 17 receptor signalling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527 (2001).
Rutitzky, L. I., Lopes da Rosa, J. R. & Stadecker, M. J. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J. Immunol. 175, 3920–3926 (2005).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice J. Immunol. 171, 6173–6177 (2003).
Bush, K. A., Farmer, K. M., Walker, J. S. & Kirkham, B. W. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 46, 802–805 (2002).
Dong, C. & Nurieva, R. I. Regulation of immune and autoimmune responses by ICOS. J. Autoimmun. 21, 255–260 (2003).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Shin, H. C. K., Benbernou, N., Fekkar, H., Esnault, S. & Guenounou, M. Regulation of IL-17, IFN-γ and IL-10 in human CD8+T cells by cyclic AMP-dependent signal transduction pathway. Cytokine 10, 841–850 (1998).
Stark, M. A. et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 (2005).
Ferretti, S., Bonneau, O., Dubois, G. R., Jones, C. E. & Trifilieff, A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170, 2106–2112 (2003).
Yao, Z. et al. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155, 5483–5486 (1995).
Infante-Duarte, C., Horton, H. F., Byrne, M. C. & Kamradt, T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 165, 6107–6115 (2000).
Hunter, C. A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nature Rev. Immunol. 5, 521–531 (2005).
Aggarwal, S., Ghilardi, N., Xie, M. H., De Sauvage, F. J. & Gurney, A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Happel, K. I. et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202, 761–769 (2005).
Khader, S. A. et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J. Immunol. 175, 788–795 (2005).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005).
Fort, M. M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Acknowledgements
I thank many colleagues for their scientific contribution to the development of the concept described in this article and supports from the National Institutes fof Health, USA, the Arthritis Foundation, USA, the Cancer Research Institute, USA, and the MD Anderson Cancer Center, USA.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Dong, C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol 6, 329–334 (2006). https://doi.org/10.1038/nri1807
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1807
This article is cited by
-
The roles of immune cells in Behçet’s disease
Advances in Rheumatology (2023)
-
Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification
Experimental & Molecular Medicine (2023)
-
The roles of metabolic profiles and intracellular signaling pathways of tumor microenvironment cells in angiogenesis of solid tumors
Cell Communication and Signaling (2022)
-
The role of the adaptive immune system and T cell dysfunction in neurodegenerative diseases
Journal of Neuroinflammation (2022)
-
Defining the TH17 cell lineage
Nature Reviews Immunology (2021)