Abstract
Background. Maspin is both overexpressed in tumors and inflammation, implicating a possible role in bridging inflammation and neoplasia. Idiopathic inflammatory bowel disease (IBD) and IBD-associated dysplasias and carcinomas represent a prototype for studying the relationship between chronic inflammatory states and neoplasia.
Aim of Study. To investigate expression of maspin in IBD and IBD-associated dysplasia and colorectal carcinoma.
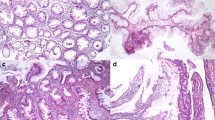
Methods. Immunohistochemical labeling of maspin was examined using tissue microarrays constructed from archival biopsy and resection tissue from 90 patients with 125 histologically defined lesions including 30 with inactive chronic IBD, 51 with active chronic IBD, 4 IBD-associated foci with epithelial changes indefinite for dysplasia (IFD), 7 with IBD-associated low-grade epithelial dysplasia (LGD), 8 with IBD-associated high grade epithelial dysplasia (HGD), and 25 with IBD-associated invasive colorectal adenocarcinomas.
Results. Maspin was expressed in 47/51 (92%) active chronic IBD lesions, which was significantly higher than both inactive chronic IBD (13/30, 43%) and normal mucosa (1 of 9, 11%) (p<0.01); in particular, the diffuse pattern of maspin expression was significantly higher in active IBD (41/51, 80%), compared with inactive IBD (5/30, 17%) and normal mucosa (0%) (p<0.01). In the multistage progression model of colitis-associated neoplasia, aberrant labeling was observed at the earliest stages, with 3/4 (75%) IFD foci, 6/7 (86%) LGD, and 8/8 (100%) HGD specimens expressing maspin, virtually always in a diffuse pattern. Expectedly, 22/25 (88%) of invasive IBD-associated cancers overexpressed maspin, including 21 with diffuse labeling.
Conclusions. Maspin is significantly overexpressed in both active IBD and colitis-associated dysplasia compared to either inactive IBD or normal colonic mucosa, suggesting a potential role in disease “flare” as well as neoplastic progression. Targeting maspin for control of disease activity and cancer prophylaxis may be a promising novel therapeutic strategy for IBD.
Similar content being viewed by others
References
Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 1994;263(5146):526–529.
Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJ, Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA 1996;93(21):11,669–11,674.
Maass N, Teffner M, Rosel F, et al. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumour progression in ductal carcinomas of the breast. J Pathol 2001;195(3):321–326.
Machtens S, Serth J, Bokemeyer C, et al. Expression of the p53 and Maspin protein in primary prostate cancer: correlation with clinical features. Int J Cancer 2001;95(5):337–342.
Xia W, Lau YK, Hu MC, et al. High tumoral maspin expression is associated with improved survival of patients with oral squamous cell carcinoma. Oncogene 2000;19(20):2398–2403.
Boltze C, Schneider-Stock R, et al. Maspin in thyroid cancer: its relationship with p53 and clinical outcome. Oncol Rep 2003;10(6):1783–1787.
Zheng HC, Wang MC, Li JY, Yang XF, Sun JM, Xin Y. Expression of maspin and kail and their clinicopathological significance in carcinogenesis and progression of gastric cancer. Chin Med Sci J 2004;19(3):193–198.
Ito R, Nakayama H, Yoshida K, Oda N, Yasui W. Loss of maspin expression is associated with development and progression of gastric carcinoma with p53 abnormality. Oncol Rep 2004;12(5):985–990.
Umekita Y, Yoshida H. Expression of maspin is up-regulated during the progression of mammary ductal carcinoma. Histopathology 2003;42(6):541–545.
Son HJ, Sohn TS, Song SY, Lee JH, Rhee JC. Maspin expression in human gastric adenocarcinoma. Pathol Int 2002;52(8):508–513.
Sood AK, Fletcher MS, Gruman LM, et al. The paradoxical expression of maspin in ovarian carcinoma. Clin Cancer Res 2002;8(9):2924–2932.
Ohike N, Maass N, Mundhenke C, et al. Clinicopathological significance and molecular regulation of maspin expression in ductal adenocarcinoma of the pancreas. Cancer Lett 2003;199(2):193–200.
Sheng S. Maspin and the order of signaling. Cancer Biol Ther 2003;2(4):404–405.
Zhang M. Multiple functions of maspin in tumor progression and mouse development. Front Biosci 2004;9:2218–2226.
Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004;287(1):G7–17.
Bettstetter M, Woenckhaus M, Wild PJ, et al. Elevated nuclear maspin expression is associated with microsatellite instability and high tumour grade in colorectal cancer. J Pathol 2005;205(5):606–614.
Song SY, Lee SK, Kim DH, et al. Expression of maspin in colon cancers: its relationship with p53 expression and microvessel density. Dig Dis Sci 2002;47(8):1831–1835.
Burmer GC, Rabinovitch PS, Haggitt RC, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology 1992;103(5):1602–10.
Itzkowitz SH. Cancer prevention in patients with inflammatory bowel disease. Gastroenterol Clin North Am 2002;31(4):1133–1144.
Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126(2):451–459.
Swierczynski SL, Maitra A, Abraham SC, et al. Analysis of novel tumor markers in pancreatic and biliary carcinomas using tissue microarrays. Hum Pathol 2004;35(3):357–366.
Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3(4):276–285.
Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis 2000;21(3):361–370.
Rachmilewitz D, Eliakim R, Ackerman Z, Karmeli F. Direct determination of colonic nitric oxide level—a sensitive marker of disease activity in ulcerative colitis. Am J Gastroenterol 1998;93(3):409–412.
Kimura H, Miura S, Shigematsu T, et al. Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn’s disease. Dig Dis Sci 1997;42(5):1047–1054.
Boughton-Smith NK, Evans SM, Hawkey CJ, et al. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet 1993;342(8867):338–340.
Khalkhali-Ellis Z, Hendrix MJ. Nitric oxide regulation of maspin expression in normal mammary epithelial and breast cancer cells. Am J Pathol 2003;162(5):1411–1417.
Nagtegaal ID, Gaspar CG, Peltenburg LT, et al. Radiation induces different changes in expression profiles of normal rectal tissue compared with rectal carcinoma. Virchows Arch 2005;446(2):127–135.
Saban R, Gerard NP, Saban MR, Nguyen NB, DeBoer DJ, Wershil BK. Mast cells mediate substance P-induced bladder inflammation through an NK (1) receptor-independent mechanism. Am J Physiol Renal Physiol 2002;283(4):F616–629.
Domann FE, Rice JC, Hendrix MJ, Futscher BW. Epigenetic silencing of maspin gene expression in human breast cancers. Int J Cancer 2000;85(6):805–810.
Boltze C, Schneider-Stock R, Quednow C, et al. Silencing of the maspin gene by promoter hypermethylation in thyroid cancer. Int J Mol Med 2003;12(4):479–484.
Murakami J, Asaumi J, Maki Y, et al. Effects of demethylating agent 5-aza-2(’)-deoxycytidine and histone deacetylase inhibitor FR901228 on maspin gene expression in oral cancer cell lines. Oral Oncol 2004;40(6):597–603.
Fitzgerald M, Oshiro M, Holtan N, et al. Human pancreatic carcinoma cells activate maspin expression through loss of epigenetic control. Neoplasia 2003;5(5):427–436.
Sato N, Fukushima N, Matsubayashi H, Goggins M. Identification of maspin and S100P as novel hypomethylation targets in pancreatic cancer using global gene expression profiling. Oncogene 2004;23(8):1531–1538.
Yatabe Y, Mitsudomi T, Takahashi T. Maspin expression in normal lung and non-small-cell lung cancers: cellular property-associated expression under the control of promoter DNA methylation. Oncogene 2004;23(23):4041–4049.
Wada K, Maesawa C, Akasaka T, Masuda T. Aberrant expression of the maspin gene associated with epigenetic modification in melanoma cells. J Invest Dermatol 2004;122(3):805–811.
Feldman RJ, Sementchenko VI, Gayed M, Fraig MM, Watson DK. Pdef expression in human breast cancer is correlated with invasive potential and altered gene expression. Cancer Res 2003;63(15):4626–4631.
Zhang M, Maass N, Magit D, Sager R. Transactivation through Ets and Ap1 transcription sites determines the expression of the tumor-suppressing gene maspin. Cell Growth Differ 1997;8(2):179–186.
Kim S, Han J, Kim J, Park C. Maspin expression is transactivated by p63 and is critical for the modulation of lung cancer progression. Cancer Res 2004;64(19):6900–6905.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, D., Wilentz, R.E., Abbruzzese, J.L. et al. Aberrant expression of maspin in idiopathic inflammatory bowel disease is associated with disease activity and neoplastic transformation. Int J Gastrointest Canc 36, 39–46 (2005). https://doi.org/10.1385/IJGC:36:1:039
Issue Date:
DOI: https://doi.org/10.1385/IJGC:36:1:039