Summary
Abstract
The treatment of irritable bowel syndrome with constipation (IBS-C) has historically been based on the severity of symptoms, with education, reassurance, dietary advice, bulking agents and laxative therapy offered as appropriate. Tegaserod (Zelnorm®, Zelmac®) is the first selective serotonin 5-HT4 receptor partial agonist to be approved for the treatment of this syndrome.
Tegaserod is active against multiple irritable bowel syndrome (IBS) symptoms; it stimulates gut motility and reduces visceral sensitivity and pain. The drug does not cure IBS and was not designed to treat the diarrhoea-predominant version. Its efficacy in men has not been established.
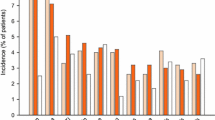
Three large well designed clinical trials of tegaserod 6mg twice daily for 12 weeks in patients (mainly women) with IBS-C have demonstrated superiority versus placebo in global relief from symptoms. Global relief response rates were 38.4–46.8% with tegaserod 6mg twice daily and 28.3–38.8% with placebo (p < 0.05–0.0001 vs placebo). The relative increases in response rates with tegaserod 6mg twice daily over the already high responses in the placebo groups ranged from 12–65% after 4–12 weeks of treatment. A response was seen within the first week. The proportion of patients with satisfactory relief from symptoms fell over the 4-week period following withdrawal of tegaserod and placebo, but did not reach baseline levels during this time.
Diarrhoea has been associated with tegaserod in clinical trials (an incidence of about 10% versus 5% with placebo, usually occurring in the first week of treatment), but the drug is otherwise well tolerated. There were no apparent changes in the tolerability profile with extended tegaserod treatment (≤12 months).
In conclusion, oral tegaserod 6mg twice daily for 12 weeks is effective and well tolerated in the treatment of IBS-C in women. Data on long term and comparative efficacy, cost-effectiveness and quality-of-life effects would be beneficial; however, in light of the fact that very few alternatives for the treatment of IBS-C have proven efficacy, tegaserod appears to be a promising option in women not responding to increased dietary fibre or osmotic laxative therapy.
Pharmacodynamic Properties
Tegaserod acts as a selective partial agonist at the serotonin 5-HT4 receptor. It binds with high affinity to human 5-HT4 receptors in vitro, but has negligible activity at other serotonin receptor subtypes or muscarinic, histaminergic, adrenergic, dopaminergic, or opiate receptors. It has one-fifth of the activity of serotonin at 5-HT4 receptors.
The effects of tegaserod on the gastrointestinal system include stimulation of motility in both the upper and lower tract, and inhibition of visceral sensitivity/ pain. In healthy men, twice daily administration of oral tegaserod 6mg for 3–14 days significantly increased the gastric emptying rate by 27–67% (p < 0.05 vs placebo); a statistically significant effect was not seen in healthy women. Small bowel transit time was shortened by 29% (p < 0.01 vs placebo) while colon transit time was reduced by 5% (p < 0.05) in both men and women. There were no gender differences in these accelerated transit times, and there was no relationship between changes in transit times and increases in faecal fluid and/or electrolyte output.
In a trial of 24 women with constipation-predominant irritable bowel syndrome (IBS-C), tegaserod 2mg twice daily did not significantly alter gastric emptying or colon transit time compared with placebo. However, a decrease in small bowel transit time was observed.
Twice daily administration of tegaserod 6mg for 7 days was effective in a clinical model of antinociception (rectal sensitivity during distention) in 20 healthy women.
Placebo-controlled trials in healthy volunteers and patients (mainly women) with IBS-C have confirmed that tegaserod at therapeutic dosages has no clinically relevant effects on the QT interval duration or any other ECG parameters.
Pharmacokinetic Properties
The absolute bioavailability of tegaserod (fasting) is approximately 10%. Mean peak plasma concentrations (Cmax) are reached approximately 1 hour after a single oral dose in healthy fasting men. Pharmacokinetic parameters are dose proportional, with no clinically relevant accumulation. The presence of food (administration 30 minutes before to 2.5 hours after a meal) reduces tegaserod Cmax and area under the plasma concentration-time curve. Time to Cmax is prolonged to 2 hours when tegaserod is administered following (but not before) food. Tegaserod is highly bound to plasma proteins (98%). The volume of distribution at steady state was 368L following intravenous administration.
Tegaserod is metabolised via two pathways. Presystemic acid-catalysed hydrolysis in the stomach followed by oxidation and glucuronidation produces the pharmacologically inactive main metabolite, 5-methoxyindole-3-carboxylic acid glucuronide (M29.0), and direct systemic glucuronidation also occurs. Following intravenous administration, the plasma clearance of tegaserod was 77 L/h and the terminal elimination half-life was approximately 11 hours. Two-thirds of an oral dose is excreted in the faeces as unchanged drug, and one-third is excreted in the urine, mainly as M29.0.
The steady-state pharmacokinetic profile of tegaserod in patients with IBS-C was similar to that in healthy volunteers, with no clinically significant effects of age, race or gender. The pharmacokinetic profile of tegaserod in patients with severe renal insufficiency was also similar to that in healthy volunteers, but the availability of M29.0 was increased. Mild hepatic insufficiency does not significantly alter availability.
Despite slight inhibition of cytochrome P450 (CYP) isoenzymes CYP1A2 and CYP2D6 in vitro, no clinically relevant interactions have been observed in clinical trials. Since the main metabolic pathway for tegaserod is presystemic non-enzymatic hydrolysis, plasma levels are unlikely to be affected by coadministered drugs.
Therapeutic Efficacy
Three large (n = 881, 1519 and 520), randomised, double-blind trials of oral tegaserod 6mg (and 2mg in one trial) given twice daily for 12 weeks to patients with IBS-C (≥83% women) have shown superiority for tegaserod over placebo. The primary efficacy endpoints in all trials were based on subjective global assessment of overall relief. In two trials, patients were asked: “Please consider how you felt this past week in regard to your irritable bowel syndrome, in particular your overall well-being, and symptoms of abdominal discomfort, pain and altered bowel habit. Compared to the way you usually felt before entering the study, how would you rate your relief of symptoms during the past week?” Response was defined as ≥50% of assessments completely or considerably relieved or 100% at least somewhat relieved in the last 4 weeks of treatment. In the third trial, Asian-Pacific patients (34% Chinese) were asked: “Over the past week, do you consider that you have had satisfactory relief from your symptoms of irritable bowel syndrome?” Response was defined as ≥75% affirmative response in the first 4 weeks of treatment.
Global relief response rates were 38.8% with tegaserod 2mg twice daily, 38.4–46.8% with 6mg twice daily and 28.3–38.8% with placebo (p < 0.05–0.0001 versus placebo). Increases in the proportion of responders with tegaserod 6mg twice daily ranged from 12% to 65% relative to placebo results over 4–12 weeks in these trials. The response to tegaserod began early (within a week) and was maintained over the 12 weeks of treatment.
The proportion of patients with satisfactory relief from symptoms fell over the 4-week period following withdrawal of tegaserod and placebo, but did not reach baseline levels in this time. Symptomatic remission was retained by more tegaserod than placebo recipients in a nonblind 8-week withdrawal study of responders to tegaserod.
Secondary endpoints (weekly assessment of abdominal pain/discomfort and constipation) were also significantly improved in tegaserod recipients compared with those receiving placebo.
Tolerability
Oral tegaserod 2 or 6mg twice daily is well tolerated in women with IBS-C. The most common tegaserod-associated adverse event reported in clinical trials was diarrhoea, which occurred at a rate of 10–11% with tegaserod (resulting in discontinuation of treatment in 1.6% of all recipients) and 4–5% with placebo. Diarrhoea usually occurred in the first 7 days of treatment, and many patients had only one episode. The overall incidence of diarrhoea was similar in patients with diarrhoea-predominant irritable bowel syndrome receiving tegaserod to that in patients receiving placebo. Continuing treatment with tegaserod over an extended period (≤12 months) in patients with IBS-C did not significantly alter the tolerability profile.
There were no clinically significant changes in laboratory or ECG analyses during 12 weeks of treatment. The addition of tegaserod treatment to low-dose antidepressant medication does not appear to increase the incidence of adverse events.
Dosage and Administration
Oral tegaserod is approved for the symptomatic treatment of IBS-C in women in many countries throughout the world. The recommended dosage is 6mg taken twice daily before a meal for one or two courses of 4–6 weeks. Treatment should be discontinued if there is no response after 4 weeks of therapy. The efficacy of tegaserod in men has not been established.
Dosage adjustments are not required for mild to moderate renal or mild hepatic impairment, but tegaserod is not indicated in patients with severe renal or moderate/severe hepatic disease. Caution is recommended in patients unable to cope with an increased incidence of diarrhoea.








Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement.
References
Forbes A. IBS: guide to diagnosis and recommended treatment. Prescriber 1997 Apr 19; 8: 67–72
Badia X, Mearin F, Balboa A, et al. Burden of illness in irritable bowel syndrome comparing Rome I and Rome II criteria. Pharmacoeconomics 2002; 20(11): 749–58
Farthing MJG. New drugs in the management of the irritable bowel syndrome. Drugs 1998 Jul; 56: 11–21
Crowell MD. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am J Manag Care 2001 Jul; 7 (8 Suppl.): S252–60
Fass R, Longstreth GF, Pimentel M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med 2001 Sep 24; 161(17): 2081–8
American Gastroenterological Association. American Gastroenterological Association medical position statement: irritable bowel syndrome. Gastroenterology 1997 Jun; 112: 2118–9
Hungin APS, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40 000 subjects. Aliment Pharmacol Ther 2003; 17: 643–50
Mayer EA, Naliboff B, Lee O, et al. Review article: gender-related differences in functional gastrointestinal disorders. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 65–9
Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut 1996; 39: 299–305
Scott LJ, Perry CM. Tegaserod. Drugs 1999 Sep; 58(3): 491–6; discussion 497-8
Bucheit K-H, Gamse R, Giger R, et al. The serotonin 5-HT4 receptor: 1. Design of a new class of agonists and receptor map of the agonist recognition site. J Med Chem 1995; 38: 2326–30
Buchheit K-H, Gamse R, Giger R, et al. The serotonin 5-HT4 receptor: 2. Structure-activity studies of the indole carbazimidamide class of agonists. J Med Chem 1995; 38: 2331–8
Norman P. Tegaserod Novartis. Current Opin Central Peripheral Nervous System Investig Drugs 2000; 2(3): 344–54
Hoyer D, Fehlmann D, Langenegger D, et al. High affinity of SDZ HTF-919 and related molecules for calf and human caudate 5-HT4 receptors. Ann N Y Acad Sci 1998 Dec 15; 861: 267–8
Appel S, Kumle A, Hubert M, et al. First pharmacokinetic-pharmacodynamic study in humans with a selective 5-hydroxytryptamine4 receptor agonist. J Clin Pharmacol 1997 Mar; 37(3): 229–37
Pfannkuche H-J, Buchheit K-H, Buhl T, et al. Substituted carbazimidamides, a new class of potent and selective 5-HT4 receptor agonists and antagonists [abstract no. 329]. Naunyn Schmiedebergs Arch Pharmacol 1996; 353 (4 Suppl.): R90
Kuemmerle JF, Murthy KS, Grider JR, et al. Human intestinal muscle cells express a rapidly desensitizing long splice variant of the 5-HT4 receptor. Gastroenterology 1996 Apr; 110 (4 Suppl.): A1091
Zimmermann AE. Tegaserod: a 5-HT4 agonist for women with constipation-predominant irritable bowel syndrome. Formulary 2002 Sep; 37: 449–61
Lacy BE. Tegaserod. Clin Perspect Gastroenterol 2002; 5(4): 251–5
Grider JR. A rapidly desensitizing 5-HT4 receptor mediates the peristaltic reflex induced by mucosal stimuli [abstract no. G3121]. Gastroenterology 1998 Apr 15; 114 (4 Suppl. Pt 2): A757
Grider JR, Foxx-Orenstein AE, Jin J-G. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 1998 Aug; 115(2): 370–80
Pfannkuche HJ, Buhl T, Gamse R, et al. The properties of a new prokinetically active drug: SDZ HTF 919. Neurogastroenterol Motil 1995 Dec; 7(4): 280
Fioramonti J, Million M, Bueno L. Investigations on a 5HT4 agonist (SDZ HTF 919) and its main metabolite in conscious dogs: effects on gastrointestinal motility and impaired gastric emptying [abstract no. G3103]. Gastroenterology 1998 Apr 15; 114 (4 Suppl. Pt 2): A752
Huge A, Zittel TT, Kreis ME, et al. Effects of tegaserod (HTF 919) on gastrointestinal motility and transit in awake rats [abstract no. 2100]. Gastroenterology 2000 Apr; 118 Suppl. 2, Pt 1: A403
Nguyen A, Camilleri M, Kost LJ, et al. SDZ HTF 919 stimulates canine colonic motility and transit in vivo. J Pharmacol Exp Ther 1997 Mar; 280(3): 1270–6
Jin J-G, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 1999 Jan; 288(1): 93–7
Appel S, Kumle A, Meier R. Clinical pharmacodynamics of SDZ HTF 919, a new 5-HT4 receptor agonist, in a model of slow colonic transit. Clin Pharmacol Ther 1997 Nov; 62(5): 546–55
Degen L, Matzinger D, Merz M, et al. Tegaserod, a 5-HT4 receptor partial agonist, accelerates gastric emptying and gastrointestinal transit in healthy male subjects. Aliment Pharmacol Ther 2001 Nov; 15(11): 1745–51
Petrig C, Templeton J, Matzinger D, et al. Effect of tegaserod on gastrointestinal transit in male and female subjects [abstract no. MON-G-512, poster]. Gut 2002; 51 Suppl. III: A138
Prather CM, Camilleri M, Zinsmeister AR, et al. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology 2000 Mar; 118(3): 463–8
Petrig C, Templeton J, Matzinger D, et al. Effect of tegaserod on faecal water and electrolyte secretion in male and female subjects [abstract no. WED-G-322, poster]. Gut 2002; 51 Suppl. III: A249
Coelho AM, Rovira P, Fioramonti J, et al. Antinociceptive properties of HTF 919 (tegaserod), a 5-HT4 receptor partial agonist, on colorectal distension in rats [abstract no. 4393]. Gastroenterology 2000 Apr; 118 Suppl. 2, Pt 1: A835
Schikowski A, Thewissen M, Mathis C, et al. Serotonin type-4 receptors modulate the sensitivity of intramural mecha-noreceptive afferents of the cat rectum. Neurogastroenterol Motil 2002 Jun; 14(3): 221–7
Camilleri M. Serotonergic modulation of visceral sensation: lower gut. Gut 2002; 51 Suppl. 1: 81–6
Coffin B, Farmachidi JP, Rueegg P, et al. Tegaserod, a 5-HT4 receptor partial agonist, decreases sensitivity to rectal distension in healthy subjects. Aliment Pharmacol Ther 2003; 17: 577–85
Dunger-Baldauf C, Rueegg PC, Lefkowitz M. Is relief from abdominal discomfort/pain in tegaserod treated IBS-C patients due to an increased frequency of bowel movements? [abstract no. 838]. Am J Gastroenterol 2002 Sep; 97 Suppl.: S275
Morganroth J, Ruegg PC, Dunger-Baldauf C, et al. Tegaserod, a 5-hydroxytryptamine type 4 receptor partial agonist, is devoid of electrocardiographic effects. Am J Gastroenterol 2002 Sep; 97(9): 2321–7
Drici MD, Ebert SN, Wang WX, et al. Comparison of tegaserod (HTF 919) and its main human metabolite with cisapride and erythromycin on cardiac repolarization in the isolated rabbit heart. J Cardiovasc Pharmacol 1999 Jul; 34(1): 82–8
Appel-Dingemanse S, Lemarechal M-O, Kumle A, et al. Integrated modelling of the clinical pharmacokinetics of SDZ HTF 919, a novel selective 5-HT4 receptor agonist, following oral and intravenous administration. Br J Clin Pharmacol 1999 May; 47(5): 483–91
Appel-Dingemanse S, Hirschberg Y, Osborne S, et al. Multiple-dose pharmacokinetics confirm no accumulation and dose proportionality of the novel promotile drug tegaserod (HTF 919). Eur J Clin Pharmacol 2001 Mar; 56(12): 889–91
Appel-Dingemanse S, Rawls J, Heggland J, et al. Similar pharmacokinetics of tegaserod (HTF 919) in patients with constipation- and diarrhea-predominant irritable bowel syndrome [abstract no. 5340]. Gastroenterology 2000 Apr; 118 Suppl. 2, Pt 2 (4): A1160
Zhou H, McLeod J, Alladina L, et al. Pharmacokinetics (PK) of SDZ HTF 919 (HTF) not altered in subjects with severe renal insufficiency requiring hemodialysis [abstract no. PIII-108]. Clin Pharmacol Ther 1999 Feb; 65(2): 203
Appel-Dingemanse S, Horowitz A, Campestrini J, et al. The pharmacokinetics of the novel promotile drug, tegaserod, are similar in healthy subjects-male and female, elderly and young. Aliment Pharmacol Ther 2001 Jul; 15(7): 937–44
Appel-Dingemanse S, Hubert M, Alladina L, et al. Pharmacokinetics and safety of SDZ HTF 919, a new promotile drug: in healthy subjects and patients with hepatic cirrhosis [abstract no. ExhB5331]. Digestion 1998; 59 Suppl. 3: 736–7
Zhou H, Khalilieh S, Lau H, et al. Effect of meal timing not critical for the pharmacokinetics of tegaserod (HTF 919). J Clin Pharmacol 1999 Sep; 39(9): 911–9
Zhou H, Khalilieh S, Campestrini J, et al. Effect of gastric pH on plasma concentrations of tegaserod (HTF 919) and its major metabolite in healthy subjects [abstract no. no. 5538]. Gastroenterology 2000 Apr; 118 Suppl. 2, Pt 2: A1206–7
Appel-Dingemanse S, Campestrini J, Rawls J, et al. Tegaserod pharmacokinetics (PK) are similar in different types of irritable bowel syndrome (IBS) and gastroesophageal reflux disease (GERD) [abstract no. A443]. J Gastroenterol Hepatol 2000 Mar; 15 Suppl.: B123
Appel-Dingemanse S. Clinical pharmacokinetics of tegaserod, a serotonin 5-HT4 receptor partial agonist with promotile activity. Clin Pharmacokinet 2002; 41(13): 1021–42
Novartis Pharmaceuticals Corporation. Zelnorm™ (tegaserod maleate) tablets; prescribing information. East Hanover (NJ): Novartis Pharmaceuticals Corporation, 2002 Jul 22
Vickers AE, Zollinger M, Dannecker R, et al. In vitro metabolism of tegaserod in human liver and intestine: assessment of drug interactions. Drug Metab Dispos 2001 Oct; 29(10): 1269–76
Zhou H, Khalilieh S, Svendsen K, et al. Tegaserod coadministration does not alter the pharmacokinetics of theophylline in healthy subjects. J Clin Pharmacol 2001 Sep; 41(9): 987–93
Kalbag J, Migoya E, Osborne S, et al. Tegaserod does not significantly affect the pharmacokinetics of dextromethorphan in healthy subjects [abstract no. 5422]. Gastroenterology 2000 Apr; 118 (4 Suppl. 2, Pt 2): A1179
Zhou H, Horowitz A, Ledford PC, et al. The effects of tegaserod (HTF 919) on the pharmacokinetics and pharmacodynamics of digoxin in healthy subjects. J Clin Pharmacol 2001 Oct; 41(10): 1131–9
Ledford P, On N, Ligueros-Saylan M, et al. Tegaserod does not significantly affect the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects [abstract no. 5445]. Gastroenterology 2000 Apr; 118 (4 Suppl. 2, Pt 2): A1184
Zhou H, Walter YH, Hubert M, et al. Tegaserod (HTF 919) does not decrease the effectiveness of an oral contraceptive when coadministered to healthy female subjects [abstract no. 5539]. Gastroenterology 2000 Apr; 118 (4 Suppl. 2, Pt 2): A1207
Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003; 52: 671–6
Müller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT4 receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 2001 Oct; 15(10): 1655–66
Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002 Nov; 16(11): 1877–88
Hamling J, Bang CJ, Tarpila S, et al. Titration regimen indicates partial 5-HT4 agonist HTF 919 improves symptoms of constipation predominant irritable bowel syndrome (C-IBS) [abstract no. ExhB5324]. Digestion 1998; 59 Suppl. 3: 735
Langaker KJ, Morris D, Pruitt R, et al. The partial 5-HT4 agonist (HTF 919) improves symptoms in constipation-predominant irritable bowel syndrome (C-IBS) [abstract no. GaPP0064]. Digestion 1998; 59 Suppl. 3: 20
Bardhan KD, Forbes A, Marsden CL. A clinical investigation of the effects of tegaserod treatment regimen on the symptoms of IBS-C in tegaserod responsive patients [abstract no. 053]. Gut 2003; 52 Suppl. 1: A14–5
Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med 1999 Nov 8; 107(5A): 91S–7S
De Ponti F, Tonini M. Irritable bowel syndrome: new agents targeting serotonin receptor subtypes. Drugs 2001; 61(3): 317–32
Poole RM. Tegaserod relieves global symptoms of irritable bowel syndrome. Inpharma 2002 Aug 10; 1350: 13–4
Novartis. Integrated summary of safety —update. 2002
Tougas G, Snape Jr WJ, Otten MH, et al. Long-term safety of tegaserod in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2002 Oct; 16(10): 1701–8
Whorwell PJ, Krumholz S, Mueller Lissner S, et al. Tegaserod has a favorable safety & tolerability profile in patients with constipation predominant and alternating forms of irritable bowel syndrome (IBS) [abstract no. 5529]. Gastroenterology 2000 Apr; 118 Suppl. 2, Pt 2: A1204
Ruegg P, Lefkowitz M, Drossman D, et al. Tegaserod alone or in combination with antidepressant drugs is well tolerated in patients with IBS-C [abstract no. 851]. Am J Gastroenterol 2002 Sep; 97 Suppl.: S279–80
Earnest D, Rueegg PC, Dunger Baldauf C, et al. Diarrhea in patients treated with tegaserod for irritable bowel syndrome with constipation (IBS-C) is infrequent and usually self-limited [abstract no. 843]. Am J Gastroenterol 2002 Sep; 97 Suppl.: S277
Plebani G, Fried M, Michetti P, et al. Safety of tegaserod in patients with irritable bowel syndrome: the Swiss experience [abstract no. WED-G-323]. Gut 2002; 51 Suppl. III: A249
Fidelholtz J, Smith W, Rawls J, et al. Safety and tolerability of tegaserod in patients with irritable bowel syndrome and diarrhea symptoms. Am J Gastroenterol 2002 May; 97(5): 1176–81
Novartis Pharmaceuticals Canada Inc. Prescribing information; Zelnorm™ (tegaserod hydrogen maleate) tablets. Dorval (Quebec): Novartis Pharmaceuticals Canada Inc., 2002 Feb 27
American Gastroenterological Association. American Gastroenterological Association medical position statement: irritable bowel syndrome. Gastroenterology 2002; 123: 2105–7
Jones J, Boorman J, Cann P, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut 2000 Nov; 47 Suppl. 2: 1–19
Talley NJ. Serotoninergic neuroenteric modulators. Lancet 2001 Dec 15; 358(9298): 2061–8
Harris MS. Irritable bowel syndrome: a cost-effective approach for primary care physicians. Postgrad Med 1997 Mar; 101(3): 215–26
Lefkowitz MP, Rueegg PC, Shi Y, et al. Validation of a global relief measure in two clinical trials of irritable bowel syndrome with tegaserod [abstract no. 855]. Gastroenterology 2000 Apr; 118 Suppl. 2, Pt 1: A145 (plus poster)
Camilleri M. Management of the irritable bowel syndrome. Gastroenterology 2001 Feb; 120(3): 652–68
Levy RL, Von Korff M, Whitehead WE, et al. Costs of care for irritable bowel syndrome patients in a health maintenance organization. Am J Gastroenterol 2001; 96(11): 3122–9
Longstreth GF, Wilson A, Knight K, et al. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol 2003; 98(3): 600–7
Leong SA, Barghout V, Birnbaum HG, et al. The economic consequences of irritable bowel syndrome: a US employer perspective. Arch Intern Med 2003; 163(8): 929–35
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: K. Bardhan, Department of General Medicine, Rotherham General Hospital, Rotherham, England; C. Beglinger, Department of Medicine, Division of Gastroenterology, University Hospital, Basel, Switzerland; J. Grider, Medical College of Virginia, Virginia Commonwealth University, Richmond, Virginia, USA; J. Kellow, Department of Medicine, The Royal North Shore Hospital, University of Sydney, St Leonards, NSW, Australia; J. Morganroth, Department of Medicine, University of Pennsylvania School of Medicine and Research Technology, Philadelphia, Pennsylvania, USA; S. Mueller-Lissner, Department of Medicine, Humboldt University, Berlin, Germany; Y. Ringel, Department of Medicine, Division of Digestive and Liver Diseases, UNC School of Medicine, Chapel Hill, North Carolina, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on tegaserod, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline, EMBASE and AdisBase search terms were ‘tegaserod’ or ‘HTF 919’. Searches were last updated 5 May 2003.
Selection: Studies in women with constipation-predominant irritable bowel syndrome who received tegaserod. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Tegaserod, irritable bowel syndrome, constipation, women, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Wagstaff, A.J., Frampton, J.E. & Croom, K.F. Tegaserod. Drugs 63, 1101–1120 (2003). https://doi.org/10.2165/00003495-200363110-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200363110-00013