Published online Feb 7, 2011. doi: 10.3748/wjg.v17.i5.666
Revised: October 22, 2010
Accepted: October 29, 2010
Published online: February 7, 2011
AIM: To evaluate the diagnostic efficacies of narrow-band imaging (NBI) endoscopy with and without high magnification in distinguishing neoplasia from non-neoplasia colorectal lesions.
METHODS: A total of 118 patients with 123 colorectal lesions examined by NBI endoscopy in the Zhejiang Provincial People’s Hospital from September 2008 to April 2010 were enrolled in this study. These lesions were classified by pit pattern and capillary pattern, and then assessed by histopathology.
RESULTS: Ten lesions not meeting the diagnostic criteria were excuded, the overall diagnostic accuracy of NBI endoscopy in distinguishing neoplasia from non-neoplasia colorectal lesions was 91.2% (103/113), and that of NBI endoscopy with and without high magnification was 93.0% (40/43) and 90.0% (63/70), respectively. Both were significantly higher than that of conventional colonoscopy reported in the literature (P < 0.05), but there was no significant difference between the two groups (P > 0.05).
CONCLUSION: Besides NBI magnifying endoscopy, NBI endoscopy without magnification may also be used to distinguish neoplasia from non-neoplasia colorectal lesions.
- Citation: Zhou QJ, Yang JM, Fei BY, Xu QS, Wu WQ, Ruan HJ. Narrow-band imaging endoscopy with and without magnification in diagnosis of colorectal neoplasia. World J Gastroenterol 2011; 17(5): 666-670
- URL: https://www.wjgnet.com/1007-9327/full/v17/i5/666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i5.666
Colorectal cancer is a common gastrointestinal malignancy with a slow process in occurrence and development involving multi-steps, multi-stages and multiple genes, and most of them arise from preexisting adenomas and have an adenoma-carcinoma sequence[1,2]. Early detection and removal of colorectal adenomas may greatly reduce both the incidence of colorectal cancer and cancer-related death[3].
Electronic colonoscopy is considered to be an effective examination for the detection of colorectal neoplastic lesions[4]. However, it is difficult to assess pre-malignant and early neoplastic lesions precisely using conventional white light endoscopy. In chromoendoscopy, a biocompatible dye, such as indigo carmine, can strengthen the surface structure of epithelial lesions[5], but the operation is relatively cumbersome, time-consuming and costly, not conducive to observe the vascular structure, and may damage the DNA of epithelial cells.
Narrow-band imaging (NBI) is a novel technology that emerged in endoscopic diagnosis of early cancer, and it has better targeting for biopsy and higher diagnostic accuracy than conventional videoendoscopy by enhancing the visualization of surface mucosal and vascular patterns on the polyp surface[6]. Our study aimed to verify the diagnostic accuracy of NBI endoscopy in distinguishing neoplasia from non-neoplasia colorectal lesions, and evaluate the diagnostic efficacies of NBI endoscopy with and without high magnification.
The patients who have poor bowel preparation, familial adenomatous polyposis, infectious bowel disease, inflammatory bowel disease and colorectal cancer were excluded. A total of 118 patients with 123 colorectal lesions examined by NBI endoscopy in the Zhejiang Provincial People’s Hospital from September 2008 to April 2010 were enrolled in this study. Forty-six and 77 lesions were examined by NBI endoscopy with and without high magnification.
A standard videoendoscopy system with two light sources was used for examination. One light source was for the standard optical filter (broadband) and the other was for the NBI system. The control knob on the grip of the endoscope allows single touch exchange of the standard filter for the NBI filter. Olympus CV-260SL, CLV-260SL, CF240I, H260AZI and SONY LMD-2140MD were used respectively for the endoscopic host, source, conventional endoscopy, magnifying endoscopy and monitor.
Researching methods, observation tables and patient’s informed consent were obtained before the study. Polyethylene glycol lavage solution and diprivan propofol were used for bowel preparation and intravenous anesthesia, respectively, and the whole examination process was managed by an experienced endoscopist (the second author). The scope was entered to the ileocecal part using conventional observation mode, and back with white light and NBI. The lesions were classified by pit pattern and capillary pattern with NBI immediately when they were detected, and then biopsied or resected for pathological diagnosis by an experienced pathologist. The endoscopist and pathologist were unaware of each other’s diagnosis, and finally the NBI endoscopic diagnosis was assessed based on the pathological diagnosis.
The NBI endoscopic diagnostic criteria followed Kudo’s[7] classification of mucosal pit pattern and Sano’s[8] classification of capillary pattern. We developed the comprehensive diagnostic criteria: Pit II + CP1 as hyperplastic polyp, Pit IIIL + CP2 or Pit IV + CP2 as adenoma, and Pit V + CP3 as adenocarcinoma.
Statistical differences of diagnostic accuracies were analyzed by the Mann-Whitney U test and χ2 test. P < 0.05 was considered significantly different.
Among the 118 patients, 73 were males and 45 were females, with a mean age of 57.54 ± 14.01 years (range, 19-86 years). The location of the lesions was as follows: 53 in rectum, 22 in sigmoid colon, 14 in descending colon, 21 transverse colon and 13 in ascending colon, with a mean size of 10.13 ± 7.79 mm (range, 3-40 mm).
Pathologically, lesions were divided into non-neoplastic lesions, including hyperplastic and inflammatory lesions (42) and neoplastic lesions, including tubular adenoma (60), villous adenoma (7) and adenocarcinoma (14).
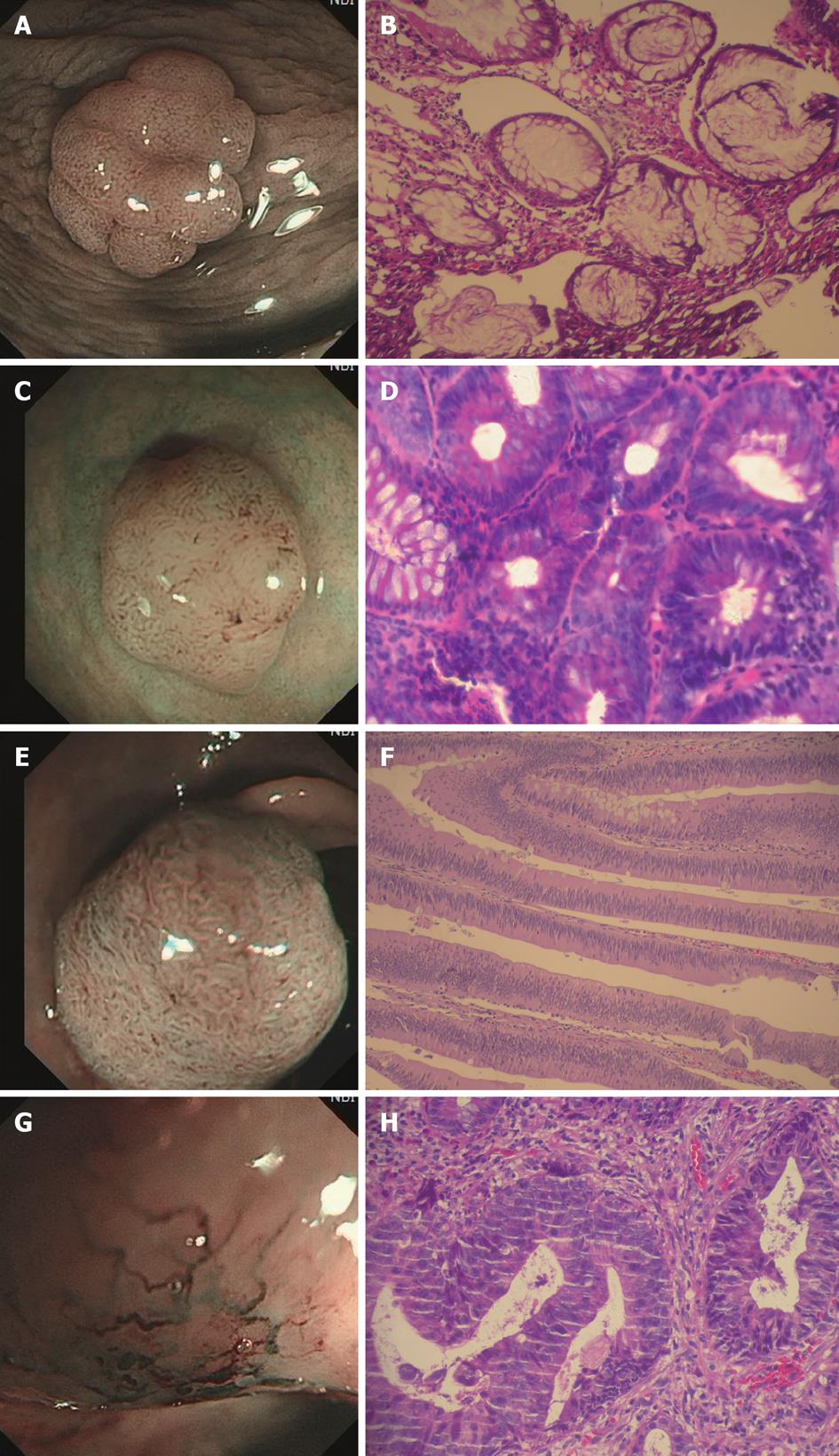
Based on the Kudo’s classification of mucosal pit pattern and Sano’s classification of capillary pattern, the lesions were classified with NBI and assessed by histopathology. The diagnostic accuracy, sensitivity and specificity to distinguish between non-neoplastic and neoplastic colorectal lesions were 94.7% (72/76), 88.9% (72/81) and 90.5% (38/42) for pit pattern delineation; and 91.7% (77/84), 95.1% (77/81) and 83.3% (35/42) for capillary pattern delineation, respectively (Table 1). According to the comprehensive diagnostic criteria, Pit II + CP1 (Figure 1A and B) was defined as hyperplastic polyp, Pit IIIL + CP2 (Figure 1C and D) or Pit IV + CP2 (Figure 1E and F) as adenoma, and Pit V + CP3 (Figure 1G and H) as adenocarcinoma. Ten lesions failed to meet the diagnostic criteria. The overall diagnostic accuracy of NBI endoscopy in distinguishing neoplastic from non-neoplastic colorectal lesions was 91.2% (103/113) and that of NBI endoscopy with and without high magnification was 93.0% (40/43) and 90.0% (63/70), respectively (Table 2). Both were significantly higher than that of conventional colonoscopy reported in the literature[9] (P < 0.05), but there was no significant difference between the two groups (P > 0.05).
| Narrow-band imaging diagnosis | Pathological diagnosis | Total | ||||
| Hyperplastic polyp | Tubular adenoma | Villous adenoma | Adenocarcinoma | |||
| Pit pattern | II | 38 | 9 | 0 | 0 | 47 |
| IIIs | 0 | 0 | 0 | 1 | 1 | |
| IIIL | 4 | 51 | 1 | 0 | 56 | |
| IV | 0 | 0 | 6 | 0 | 6 | |
| V | 0 | 0 | 0 | 13 | 13 | |
| Capillary pattern | CP1 | 35 | 4 | 0 | 0 | 39 |
| CP2 | 7 | 56 | 7 | 0 | 70 | |
| CP3 | 0 | 0 | 0 | 14 | 14 | |
| Pathological diagnosis | NBI without magnification | NBI with magnification | ||
| Consistent | Inconsistent | Consistent | Inconsistent | |
| Hyperplastic polyp | 21 | 3 | 12 | 1 |
| Adenoma | 37 | 4 | 20 | 2 |
| Adenocarcinoma | 5 | 0 | 8 | 0 |
| Total | 63 | 7 | 40 | 3 |
NBI is a novel imaging technology that uses special narrow-band filters in the endoscopic system, which allow for a more detailed visualization of the mucosal architecture and vascular pattern. Current NBI technology limits the mucosal surface light penetration, thereby enhancing the visualization of the fine capillary vessel structure on the surface layer[10]. According to a previous pilot study by Machida et al[9], NBI with magnifying endoscopy achieved better visualization of the mucosal vascular network pattern than conventional white light imaging, and the diagnostic accuracy was higher than that of conventional colonoscopy and equivalent to chromoendoscopy. Furthermore, compared with chromoendoscopy, the NBI observation has the advantage of convenient application without the necessity of dye spraying, thus the procedure can be shortened in time and an overlooked lesion with accumulation of dark-blue dye at the dependent portion of colon can also be avoided[11].
According to previous pathological studies, most of the colorectal cancers arise from preexisting adenomas and such an adenoma-carcinoma sequence, and the adenoma shares many architectural features with the carcinoma in terms of vascular architecture including vessel diameter and spatial distribution which is considerably different from that in the non-neoplastic portion of colonic mucosa[12]. Therefore, NBI endoscopy has a significant advantage in the diagnosis of colorectal dysplasia accompanied with microvascular changes. In recent years, a number of researches[9,13-15] have shown that the diagnostic accuracy of NBI endoscopy in distinguishing neoplastic from non-neoplastic colorectal lesions was higher than that of conventional colonoscopy and equivalent to chromoendoscopy. In our study, we used Kudo’s classification of mucosal pit pattern and Sano’s classification of capillary pattern, and the diagnostic accuracy, sensitivity and specificity to distinguish between non-neoplastic and neoplastic colorectal lesions were 94.7% (72/76), 88.9% (72/81) and 90.5% (38/42) for pit pattern delineation, which is similar to the data reported in the literature (92.7%, 95.7% and 87.5%), significantly higher than that of conventional endoscopy (82.9%, 80.0% and 81.8%)[14]; and 91.7% (77/84), 95.1% (77/81), 83.3% (35/42) for capillary pattern delineation, which is slightly lower than the data reported in the literature (96.6%, 97.1% and 91.8%)[16], but there was no significant difference. It may be related to the relatively small number of the cases in this study. In addition, it is possible that endoscopists who are familiar with NBI endoscopy may also improve the diagnostic accuracy, but this requires further investigation.
In recent years, as NBI combined with magnifying endoscopy could enhance the contrast detailed morphological changes in the mucosal surface and clearly visualize the microvascular structures, most studies described the use of NBI endoscopy with magnification[13,17-22], but few data about diagnostic accuracy of NBI endoscopy without magnification were reported. However, magnifying endoscopy is not clinically used as standard endoscopic equipment in most institutions, not only in China but also in Japan and some Western countries, because magnifying endoscopy is much more expensive. This greatly restricted the wide application of NBI magnifying endoscopy. In order to evaluate the diagnostic efficacies of NBI conventional endoscopy in distinguishing neoplastic from non-neoplastic colorectal lesions, we compared the diagnostic accuracy of NBI with and without high magnification. The results showed that the overall diagnostic accuracy of NBI endoscopy in distinguishing neoplastic from non-neoplastic colorectal lesions was 91.2% (103/113), and that of NBI endoscopy with and without high magnification was 93.0% (40/43) and 90.0% (63/70), respectively. Both were significantly higher than that of conventional colonoscopy reported in the literature, but there was no significant difference between the two groups. Therefore, we believe that NBI endoscopy without high magnification could also greatly improve the diagnostic accuracy and may also be used to distinguish neoplastic from non-neoplastic colorectal lesions instead of NBI magnifying endoscopy. However, as the sample was relatively small in our study, it was not clear whether NBI without high magnification could improve the detection rate in the mass population screening; this requires further study and investigation.
As a new non-invasive endoscopic method, the diagnostic efficacies of NBI combined with magnifying endoscopy in distinguishing neoplastic from non-neoplastic colorectal lesions has been confirmed by extensive literatures[13,14,23-25]. We found in our study that the endoscopic system installed with the NBI system enables the clinicians to significantly improve the diagnostic accuracy. Therefore, even the institutions without the expensive magnifying endoscopy equipment can also use conventional NBI endoscopy to get an accurate diagnosis. Although these findings need to be confirmed in large prospective trials, this initial experience with conventional NBI endoscopy is encouraging and holds promise for future application in prospective studies. In addition, endoscopists, through training in NBI endoscopic practice, may also improve their own diagnostic accuracy and raise the detection rate of colorectal neoplasia[26].
Narrow-band imaging (NBI) is a novel technology developed for endoscopic diagnosis of early cancer, and it has a higher diagnostic accuracy than conventional endoscopy by enhancing the visualization of surface mucosal and vascular patterns on the lesion surface.
In recent years, as NBI combined with magnifying endoscopy could enhance the contrast detailed morphological changes in the mucosal surface and clearly visualize the microvascular structures, most studies described the use of NBI endoscopy with magnification, but few data about diagnostic accuracy of NBI endoscopy without magnification were reported.
Compared with NBI magnifying endoscopy, NBI endoscopy without magnification may be used in distinguishing neoplasia from non-neoplasia colorectal lesions.
NBI endoscopy enables clinicians to significantly improve their diagnostic accuracy. Even those institutions equipped without magnifying endoscope can also use NBI conventional endoscopy to get an accurate diagnosis.
NBI is a novel imaging technology that uses special narrow-band filters in the endoscopic system, which allow for a more detailed visualization of the mucosal architecture and vascular pattern.
This paper compares narrow band imaging with and without high magnification in 100 patient with colorectal lesions over an 18 mo period. The authors show that both of these techniques are more accurate than conventional colonoscopy in distinguishing between neoplastic and non-neoplastic lesions with similar sensitivity. A larger study would have had more value.
Peer reviewers: Dr. Benjamin Perakath, Professor, Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India; Chakshu Gupta, MD, FCAP, Pathology and Laboratory Medicine, Heartland Regional Medical Center, 5325 Faraon Street, St. Joseph, MO 64506, United States; Dr. John B Schofield, MB, BS, MRCP, FRCP, Department of Cellular Pathology, Preston Hall, Maidstone, Kent, ME20 7NH, United Kingdom
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Allen JI. Molecular biology of colon polyps and colon cancer. Semin Surg Oncol. 1995;11:399-405. [Cited in This Article: ] |
| 2. | Carethers JM. The cellular and molecular pathogenesis of colorectal cancer. Gastroenterol Clin North Am. 1996;25:737-754. [Cited in This Article: ] |
| 3. | Niv Y, Dickman R, Figer A, Abuksis G, Fraser G. Case-control study of screening colonoscopy in relatives of patients with colorectal cancer. Am J Gastroenterol. 2003;98:486-489. [Cited in This Article: ] |
| 4. | Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, Yee J, Henderson J, Hatten P, Burdick S. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305-311. [Cited in This Article: ] |
| 5. | Hurlstone DP, Fujii T. Practical uses of chromoendoscopy and magnification at colonoscopy. Gastrointest Endosc Clin N Am. 2005;15:687-702. [Cited in This Article: ] |
| 6. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [Cited in This Article: ] |
| 7. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [Cited in This Article: ] |
| 8. | Ikematsu H, Matsuda T, Emura F, Saito Y, Uraoka T, Fu KI, Kaneko K, Ochiai A, Fujimori T, Sano Y. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010;10:33. [Cited in This Article: ] |
| 9. | Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094-1098. [Cited in This Article: ] |
| 10. | Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kwon R, Mamula P, Rodriguez B, Shah RJ. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581-589. [Cited in This Article: ] |
| 11. | Emura F, Saito Y, Ikematsu H. Narrow-band imaging optical chromocolonoscopy: advantages and limitations. World J Gastroenterol. 2008;14:4867-4872. [Cited in This Article: ] |
| 12. | Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354-1362. [Cited in This Article: ] |
| 13. | Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988-995. [Cited in This Article: ] |
| 14. | Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711-2716. [Cited in This Article: ] |
| 15. | Chang CC, Hsieh CR, Lou HY, Fang CL, Tiong C, Wang JJ, Wei IV, Wu SC, Chen JN, Wang YH. Comparative study of conventional colonoscopy, magnifying chromoendoscopy, and magnifying narrow-band imaging systems in the differential diagnosis of small colonic polyps between trainee and experienced endoscopist. Int J Colorectal Dis. 2009;24:1413-1419. [Cited in This Article: ] |
| 16. | Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278-283. [Cited in This Article: ] |
| 17. | Horimatsu T, Sano Y, Kaneko K, Ikematsu H, Katagiri A, Yano T, Fu KI, Muto M, Fujii S, Ochiai A. Relationship between MVD and meshed-capillaries using magnifying NBI colonoscopy in colorectal precursor lesions. Hepatogastroenterology. 2009;56:372-377. [Cited in This Article: ] |
| 18. | Kanao H, Tanaka S, Oka S, Hirata M, Yoshida S, Chayama K. Narrow-band imaging magnification predicts the histology and invasion depth of colorectal tumors. Gastrointest Endosc. 2009;69:631-636. [Cited in This Article: ] |
| 19. | Katagiri A, Fu KI, Sano Y, Ikematsu H, Horimatsu T, Kaneko K, Muto M, Yoshida S. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:1269-1274. [Cited in This Article: ] |
| 20. | Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc. 2007;66:945-952. [Cited in This Article: ] |
| 21. | Muto M, Horimatsu T, Ezoe Y, Morita S, Miyamoto S. Improving visualization techniques by narrow band imaging and magnification endoscopy. J Gastroenterol Hepatol. 2009;24:1333-1346. [Cited in This Article: ] |
| 22. | Uraoka T, Saito Y, Matsuda T, Sano Y, Ikehara H, Mashimo Y, Kikuchi T, Saito D, Saito H. Detectability of colorectal neoplastic lesions using a narrow-band imaging system: a pilot study. J Gastroenterol Hepatol. 2008;23:1810-1815. [Cited in This Article: ] |
| 23. | Fukuzawa M, Saito Y, Matsuda T, Uraoka T, Itoi T, Moriyasu F. Effectiveness of narrow-band imaging magnification for invasion depth in early colorectal cancer. World J Gastroenterol. 2010;16:1727-1734. [Cited in This Article: ] |
| 24. | Rastogi A, Pondugula K, Bansal A, Wani S, Keighley J, Sugar J, Callahan P, Sharma P. Recognition of surface mucosal and vascular patterns of colon polyps by using narrow-band imaging: interobserver and intraobserver agreement and prediction of polyp histology. Gastrointest Endosc. 2009;69:716-722. [Cited in This Article: ] |
| 25. | Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373-379. [Cited in This Article: ] |
| 26. | Adler A, Pohl H, Papanikolaou IS, Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC, Setka E, Koch M, Wiedenmann B. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut. 2008;57:59-64. [Cited in This Article: ] |