Published online Sep 28, 2013. doi: 10.3748/wjg.v19.i36.6011
Revised: July 17, 2013
Accepted: July 23, 2013
Published online: September 28, 2013
AIM: To report outcomes on patients undergoing radiofrequency ablation (RFA) for early oesophageal squamous neoplasia from a National Registry.
METHODS: A Prospective cohort study from 8 tertiary referral centres in the United Kingdom. Patients with squamous high grade dysplasia (HGD) and early squamous cell carcinoma (ESCC) confined to the mucosa were treated. Visible lesions were removed by endoscopic mucosal resection (EMR) before RFA. Following initial RFA treatment, patients were followed up 3 monthly. Residual flat dysplasia was treated with RFA until complete reversal dysplasia (CR-D) was achieved or progression to invasive Squamous cell cancer defined as infiltration into the submucosa layer or beyond. The main outcome measures were CR-D at 12 mo from start of treatment, long term durability, progression to cancer and adverse events.
RESULTS: Twenty patients with squamous HGD/ESCC completed treatment protocol. Five patients (25%) had EMR before starting RFA treatment. CR-D was 50% at 12 mo with a median of 1 RFA treatment, mean 1.5 (range 1-3). Two further patients achieved CR-D with repeat RFA after this time. Eighty per cent with CR-D remain dysplasia free at latest biopsy, with median follow up 24 mo (IQR 17-54). Six of 20 patients (30%) progressed to invasive cancer at 1 year. Four patients (20%) required endoscopic dilatations for symptomatic structuring after treatment. Two of these patients have required serial dilatations thereafter for symptomatic dysphagia with a median of 4 dilatations per patient. The other 2 patients required only a single dilatation to achieve an adequate symptomatic response. One patient developed cancer during follow up after end of treatment protocol.
CONCLUSION: The role of RFA in these patients remains unclear. In our series 50% patients responded at 12 mo. These figures are lower than limited published data.
Core tip: Squamous cell cancer of the esophagus is an aggressive pathology with a poor prognosis and therefore early intervention is paramount to improve survival. Minimally invasive endoscopic therapy with endoscopic resection and radiofrequency ablation in patients with early squamous neoplasia of the esophagus has the potential to treat selected patients. Early response to RFA is prognostically important. Further studies with more patient numbers are required to explore the long and short term efficacy of this intervention in these complex patients.
- Citation: Haidry RJ, Butt MA, Dunn J, Banks M, Gupta A, Smart H, Bhandari P, Smith LA, Willert R, Fullarton G, John M, Pietro MD, Penman I, Novelli M, Lovat LB. Radiofrequency ablation for early oesophageal squamous neoplasia: Outcomes form United Kingdom registry. World J Gastroenterol 2013; 19(36): 6011-6019
- URL: https://www.wjgnet.com/1007-9327/full/v19/i36/6011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i36.6011
Squamous cell cancer (SCC) comprises nearly 90% of all esophageal cancers worldwide[1]. The incidence of esophageal SCC has fallen in the western world in the past 3 decades but still remains between 4 and 16 per 100000 population. This is strongly dependent on geographical location worldwide with figures far higher in Asia[2]. In the western world factors such as alcohol and tobacco play an important role in the development of oesophageal SCC[3]. This condition caries a poor prognosis with an overall five year survival rate of 10%-15%[4]. Those treated with surgery following neo-adjuvant therapy still carry a poor prognosis with a 5 year overall survival of about 33%. Surgery carries a significant mortality of 5% and postoperative morbidity of up to 47%[5]. The precursor lesion to SCC is known as squamous dysplasia. The World Health Organization (WHO) refers to squamous dysplasia as squamous intra-epithelial neoplasia and has further categorized the condition depending on the grade of dysplasia as low grade intra-epithelial neoplasia (LGIN) through to high grade intra-epithelial neoplasia (HGIN)[6]. Non invasive SCC is often referred to as ESCC.
Squamous high-grade dysplasia and ESCC carry a risk of progression to invasive SCC of up to 65% at 3.5 years and as high as 74% at 13.5 years[7]. The chance of lymph node metastasis is dependent upon the penetration and depth of the lesion. Lesions restricted to the epithelial layer (mL) or the lamina propria (m2) have a low rate of lymph node metastases (< 5%); lesions that penetrate into the muscularis mucosae (m3) or the first third of the submucosa (sm1) have a higher risk (5%-15%)[8-12]. Once there is deeper submucosal (sm2 and sm3) involvement the risk of lymph node spread can be in the region of 24%[13,14] and surgical or oncological interventions are the treatments of choice. Traditional treatment for squamous neoplasia has been surgery or chemo-radiotherapy. However with disease limited to the mucosa the risk of lymph node involvement is low and minimally invasive endoscopic therapy is an alternative.
With the advances in minimally invasive esophageal endotherapy over the past decade there are now additional treatment options for patients with squamous HGD and ESCC confined to the lamina propria. Centers in Asia and the Western countries have shown that the use of EMR is effective and curative for these patients[15,16]. However this form of endotherapy is associated with significant oesophageal stenosis[17] and therefore alternative treatments have been desirable. The use of photodynamic therapy in this cohort of patients has again shown promising results[18] but is not widely available and again is associated with significant stenosis post treatment.
Endoscopic submucosal dissection (ESD) has been developed in Asia as one of the standard endoscopic resection techniques for early squamous neoplasia of the oesophagus. ESD enables oesophageal lesions, regardless of their size, to be removed en bloc and thus has a lower local recurrence rate than EMR. The en bloc resection rate is greater than 90% (90.6%-100%)[19-25]. En bloc resection, meaning resection in a single piece, facilitates an accurate histological assessment and reduces the risk of recurrence. In fact, the local recurrence rate after oesophageal ESD is extremely low (0%-3.1%)[21-25]. ESD is yet to become established in the united kingdom as this techniques is not widely available and is confined to specialist center’s only.
RFA using the HALO system (BÂRRX Medical, Sunnyvale, California, United States) is a novel minimally invasive field ablation technique which has established efficacy for treating HGD and early adenocarcinoma arising in Barrett’s esophagus (BE)[26]. The HALO System uses ultra short pulsed radiofrequency that ablates the mucosa whilst preserving the submucosa. Emerging evidence suggests that RFA is safe and efficacious in the management of squamous HGD[26-29]. We report the United Kingdom HALO registry experience of the first 20 patients with squamous HGD and ESCC who have completed treatment protocol.
The UK HALO RFA registry was created to audit outcomes of patients undergoing RFA for HGD/early neoplasia in BE and patients with squamous esophageal HGD/ESCC. It is a prospective multicenter registry which holds patient data from 20 centers nationwide.
Ethical approval was granted by the Joint UCL/UCLH Committee on the ethics of Human research (REC REF 08/H0714/27). The HALO ablation system has already been approved by the US food and Drug Administration (FDA). In addition it is a European Cleared (CE) device as well as having been approved by the National Institute of Clinical Excellence (NICE) in the United Kingdom for treatment of HGD in BE.
All patients referred for consideration for endotherapy for squamous HGD and early SCC were invited to enter at collaborating centers. Patients had an endoscopic and histological diagnosis of squamous HGD or ESCC confirmed by two independent expert gastrointestinal histopathologist prior to embarking on endotherapy.
Between January 2008 and March 2013 a total of 670 patients were enrolled to the registry from a total of 20 centers nationwide in the United Kingdom. Amongst these, 27 patients from 8 centers had squamous HGD or ESCC. Twenty of these have completed treatment protocol.
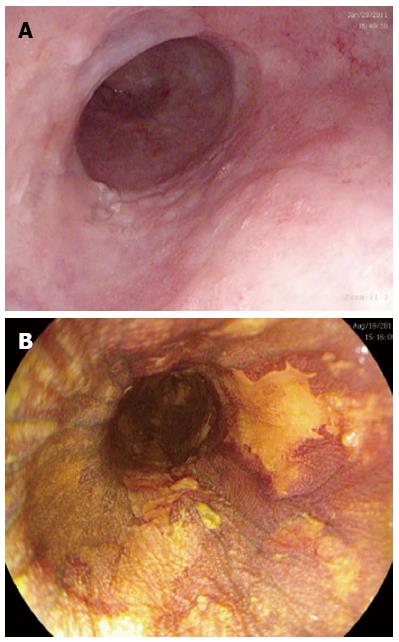
All patients were referred by the cancer center specialist multidisciplinary team (sMDT). All had endoscopic assessment with multiple biopsies to exclude invasive disease. All investigators used the Paris Classification to classify macroscopic lesions[30]. Enhanced endoscopic techniques including chromoendoscopy with Lugol’s iodine, narrow band imaging (Olympus, Hamburg, Germany) and I-scan (Pentax, Hoya Corporation, Japan) were used to target areas of suspected squamous dysplasia (see Figure 1) depending on which technology was present in the respective hospitals. Endoscopic ultrasound (EUS) and CT scanning was performed according to sMDT requirements. Endoscopic ultrasound was used if there were visible lesions seen to ensure that the neoplasia was confined to the mucosa. Endoscopic mucosal resection (EMR) was performed for any raised visible lesions and assessed by two expert gastrointestinal histopathologists at each center. Four quadrant biopsies were then obtained very 2 cm through the oesophagus to map for any further neoplasia. If invasive cancer was detected, the patient was referred back to the sMDT for alternative therapy. Invasive cancer was defined as neoplasia invading into the submucosa such that it was no longer amenable to endoscopic therapy with EMR or RFA.
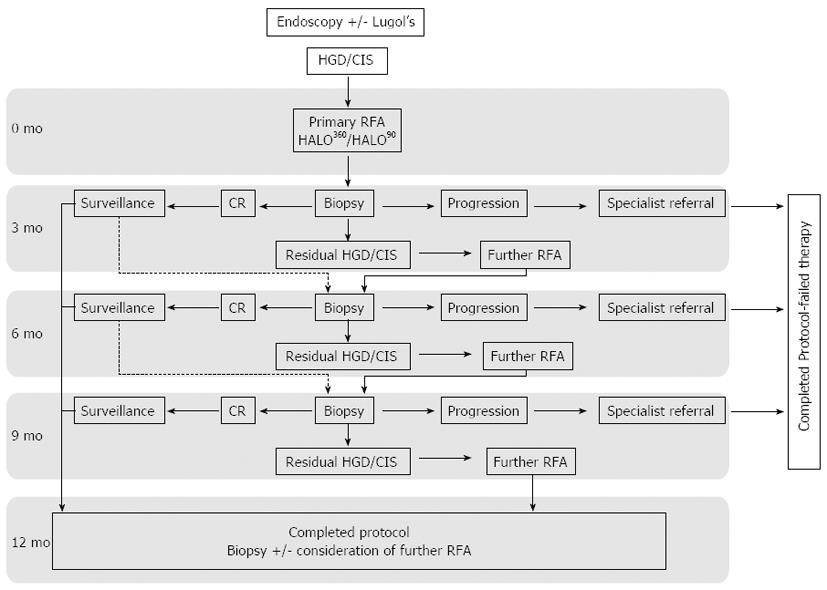
Once consented patients had their first ablation as described below. At 3 mo all patients returned for follow up endoscopy where mapping biopsies were taken using chromoendoscopy with Lugol’s iodine and enhanced endoscopic imaging. As well as targeted biopsies of USLs seen after Lugol’s staining, systematic 4 quadrant biopsies every 2 cm were taken through the squamous esophagus to map any residual dysplasia. Any new raised visible lesions were treated with EMR. A decision to repeat RFA treatment was based on histology rather than visual clearance of disease. At 12 mo after the first ablation, biopsies were again taken to assess for eradication of dysplasia (CR-D) and this was defined as the primary end point. Patients with residual disease at this point were considered for ongoing endotherapy at the clinicians’ discretion after consultation with the patient and discussion at the sMDT. Development of invasive cancer at any time was defined as treatment failure and data were censored at this point. An overview of the study protocol is shown in Figure 2.
RFA was delivered circumferentially (HALO 360) or focally (HALO 90) at 12 J/cm2. As opposed to the 2 ablations delivered for RFA in BE at each treatment session, only a single ablation was delivered in the registry protocol for squamous HGD. In patients with multifocal dysplasia circumferential RFA was applied and focal RFA administered in patients with unifocal well defined areas of dysplasia. These areas were reported in centimeters from the incisors so that follow up procedures could use this reference to interrogate treated segments of esophagus for success or failure.
Chromoendoscopy with magnifying endoscopy and enhanced imaging were used at follow up procedures to examine the previously treated areas referenced at the previous endoscopy. Again all histology was reviewed by two expert gastrointestinal histopathologists.
All patients were maintained on a twice-daily regimen of a proton pump inhibitor. Soluble co-codamol was prescribed for discomfort post procedure. All patients were discharged home the same day after review by the endoscopist. Follow up endoscopies were carried out at 3 monthly intervals as per protocol with enhanced endoscopic imaging and lugol’s chromoendoscopy (Figure 2).
The primary end points were complete reversal of squamous dysplasia (CR-D) at 12 mo and development of invasive cancer at any stage. Secondary end points include long-term durability, number of RFA procedures and adverse events. We also followed up all patients who progressed to cancer so that we could determine their long-term outcomes.
Endpoints such as CR-D at end of protocol were compared to the patient’s baseline status using log rank test and long term outcomes predicted with Kaplan-Meier survival analysis.
Twenty seven patients were treated at 8 different centers nationwide. Twenty of these have completed the treatment protocol and we report the outcomes of these patients.
Pre treatment parameters are given in Table 1. Four patients (20%) had disease limited to 1cm (focal disease) and the remaining 16 patients had definable lengths of dysplasia (multi-focal disease) with a median length of 5cm (IQR 1-10). Five patients (25%) had EMR before starting RFA treatment. All patients gave informed written consent. Median follow up for those who had successful ablation and are still in follow up (n = 10) is 24 mo (IQR 17-54).
| Variable | Value |
| No. of patients completed protocol | 20 |
| Age, yr | |
| mean ± SD | 71.6 ± 9.4 |
| Range | 38-88 |
| Sex, | |
| Female | 16 (80) |
| Male | 4 (20) |
| Caucasian ethnicity, | 20 (100) |
| Grade of esophageal neoplasia at study entry | |
| High grade dysplasia (Tis) | 12 (60) |
| ESCC (T1a) | 8 (40) |
| 1Focal squamous dysplasia | 4 (20) |
| 2Multi-focal squamous dysplasia | 16 (80) |
| Length of non-focal squamous dysplasia, cm | |
| mean ± SD | 6.1 ± 4.2 |
| Number of centers recruiting | 8 |
| Previous endoscopic mucosal resection | 5 (25) |
Patients had a median of 1 RFA session and mean of 1.5 RFA treatments during the protocol period. A total of 6 rescue EMRs have been carried out in 6 patients after their first RFA. Two of these patients had already undergone EMR prior to initiating RFA treatment.
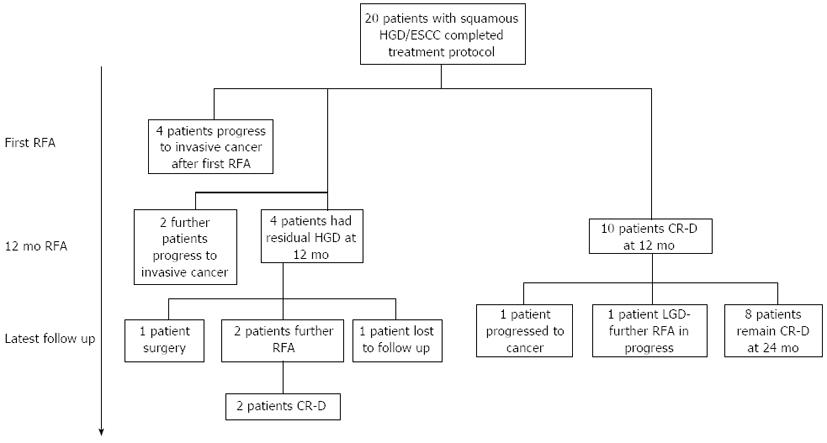
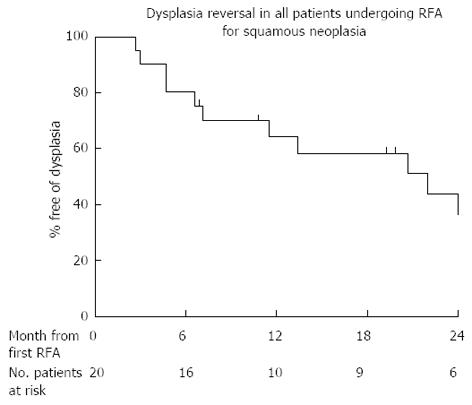
Ten patients (50%) had reversal of dysplasia/CIS (CR-D) at end of protocol after a median of 1 treatment. Eight of these patients (80%) remain free of dysplasia on their latest follow up (median follow up from first treatment 24 mo, range 19-54 mo, see Figure 3). Of the 2 patients who had a recurrence after initial successful RFA, one progressed to invasive disease. The other had multifocal low grade dysplasia (LGD) 4 mo after completing treatment and at latest follow up (41 mo after protocol end) has had 3 further circumferential ablations and one focal ablation. This patient still has LGD at latest follow up. The clinician and patient have, nonetheless, agreed to perform a further RFA treatment.
Four patients (20%) had residual dysplasia at 12 mo. One patient was referred for surgery to remove the dysplasia. One patient left the country and has been lost to follow up. The other 2 have opted to have further RFA and after a mean of 2 RFA treatments over 10 mo are free of disease at present (Figure 3). Figure 4 shows the predicted rate of dysplasia reversal in all 20 patients who have undergone treatment and completed protocol using Kaplan Meier outcome statistics.
Although our numbers are too small to draw firm conclusions, there is a trend towards baseline histological grade (HGD or ESCC) having an influence on eventual outcome with HGD having a better outcome (66% vs 33%, HR = 0.535, P = 0.0308, 95%CI: 0.141-1.505, Log rank test). Larger numbers are however needed to confirm this trend. Our data do not suggest that EMR before commencing RFA influences dysplasia reversal rates and long term outcomes (33% vs 66%, HR = 0.229, P = 0.778, 95%CI: 0.3264-3.642, P = 0.8882, Log Rank test). Dysplasia reversal was also the same whether EMR was required during the RFA protocol (3/6 - 50%) or whether patients underwent RFA alone (7/14 - 50%).
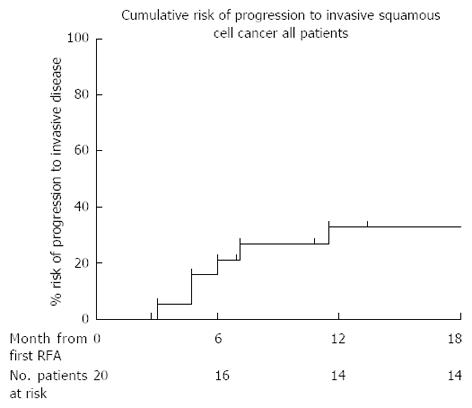
Four of the 20 (20%) patients had progressed to invasive SCC at their first follow up and therefore no further RFA was performed. Two further patients who were treated with an initial circumferential RFA followed by an EMR at follow up endoscopy progressed to invasive cancer at protocol end. All progressors were referred for consideration of chemoradiotherapy. Using Kaplan Meier analysis, the risk of progression to invasive disease in all 20 patients who completed the protocol is 26% at 18 mo (Figure 5).
One patient suffered a superficial esophageal tear following sizing prior to attempted circumferential ablation. The procedure was discontinued and focal ablation was used at the same procedure. The patient was discharged home without any complications. Four patients (20%) required dilatations for moderate esophageal structuring after their first circumferential treatment. Only one had been treated by EMR prior to RFA. Two of these patients have required serial dilatations thereafter for symptomatic dysphagia with a median of 4 dilatations per patient. The other 2 patients required only a single dilatation to achieve an adequate symptomatic response. Three serious adverse events have been reported to date. Two patients had bleeding at their follow up endoscopy after biopsies and required adrenaline injection. Both occurred following Lugol’s iodine application. Although they were admitted overnight in hospital for observation they were discharged the following day without blood transfusion.
The role for endotherapy such as RFA as a first line intervention for patients with squamous HGD and ESCC in situ is yet to be established as standard practice. Squamous dysplasia is a very aggressive pathology and early diagnosis and intervention are paramount as disease progression often precludes curative therapy. However the high surgical mortality rate of up to 2%-5% and subsequent morbidity of 20%-50% means that alternative minimally invasive and novel techniques must be investigated[5,11,12,31].
Early literature into the use of RFA in squamous dysplasia emerged in 2008, following on from its recognized potential in early Barrett’s neoplasia. Pouw et al[32] described the case of a 66-year-old patient with a unifocal lesion within the esophagus. This lesion had arisen after the patient had previously undergone chemoradiotherapy for a T2N1M0 squamous cell cancer of the hypopharynx. Following pre-treatment staging to ensure the lesion was confined to the mucosal surface only, the patient received a single balloon based ablation and had no recurrent disease at 4 mo follow up. Subsequent to this report, data regarding the efficacy of RFA in squamous dysplasia have been limited. One of the largest series to date examined the success of RFA in 13 patients within two tertiary centers[28]. Nine of the study cohort (69%) required EMR at baseline for visible nodules prior to commencing RFA. Dysplasia reversal was excellent in these patients with 100% achieving CR-D with a median of 2 treatments and remaining disease free with a follow up period of 17 mo from first treatment. In this study patients received 2 ablations at each treatment endoscopy with 12 J/cm2 for the circumferential ablation and 15 J/cm2 for focal ablation. Stenosis and stricturing was confined to just 2 of the 13 patients in this series. This same group has recently gone on investigate the efficacy of RFA in a larger cohort of 29 patients in a prospective study[33]. This study was conducted in a single Chinese center. All patients underwent an index circumferential ablation of all unstained lesions (USLs) with Lugol’s chromoendoscopy. All patients were followed up at 3 monthly intervals with chromoendoscopy with biopsies followed by focal ablation of any USLs. Using the Chinese classification of squamous neoplasia[34], 18 patients had moderate intraepithelial neoplasia (MGIN), 10 had high grade intraepithelial neoplasia (HGIN), and a single patient had early ESCC. At 12 mo 97% (28/29) had a complete reversal of neoplasia and furthermore there was no progression to invasive cancer within the treated group. The single patient with residual disease at 12 mo had EMR for unifocal disease with clear resection margins.
In our study examining outcomes from the UK RFA registry of patients with squamous dysplasia undergoing RFA, CR-D was 50% at protocol completion, although dysplasia was later reversed in two further patients following more RFA sessions. These figures are lower than the limited published data from other centers worldwide[28]. We designed our study with a protocol end at 12 mo so that this study could be directly compared with those previously published. It may be that future studies should consider alternative end points to allow for a longer duration of treatment.
In our series 20% of patients progressed to invasive disease after only one session of RFA and were then offered chemo-radiotherapy. These patients represent most of those who eventually developed cancer. This suggests that a single RFA treatment might even be considered as a staging procedure. Early failure would identify patients who should be treated with more conventional modalities. Indeed, 80% of those who achieved successful reversal of dysplasia at the end of the RFA treatment protocol remain in remission at most recent follow up.
The 20% rate of progression after a single treatment may also point to the fact that these patients may have been under staged and may in some cases harbor more aggressive neoplasia at baseline that was not sampled. Whereas with other dysplastic conditions of the esophagus such as BE where there are often distinct areas of abnormality within the macroscopically visible columnar lined mucosa, with squamous dysplasia these areas are subtle. Detection relies on adjuncts such as chromoendoscopy with Lugol’s iodine solution, optical endoscopic enhancements and the experience and expertise of the endoscopists to spot anomalies. Even with Lugol’s solution the accuracy of detecting lesions varies greatly. In a recently published series the positive predictive value for Lugol’s detecting squamous neoplasia in unstained lesions after RFA was only 14%[33].
It appears that the use of EMR in our cohort of patients is somewhat limited compared to similar published studies[15,35,36]. Only 5 patients (25%) underwent EMR before starting HALO RFA and there were a total of only 6 resections in 4 patients after their index treatment. This may account for the lower rates of dysplasia clearance and progression after the index treatment. Visible nodular lesions before or after RFA treatment may harbor submucosal disease and unless resected early may represent recurrence and progressive invasive disease.
The published data on the success of RFA in BE is robust and plentiful whilst there is limited data on its use in squamous HGD. The AIM dysplasia trial[26] demonstrated impressive outcomes with reversal of HGD in patients with BE as high as 81% with a structured ablation protocol over 12 mo. In this protocol all patients were ablated twice at each treatment with 12 J/cm2 for both circumferential and focal ablation. Mean treatments per patient was 3.5 ablations and stricturing occurred in 5% requiring dilatations. Subsequent published literature and our own outcomes from the RFA United Kingdom national registry have reproduced similar outcomes in patients with BE[37]. In 335 patients with Barrett’s related neoplasia, HGD was cleared from 86% of patients, all dysplasia from 81%, and BE from 62%, at the 12-mo time point, following a mean 2.5 RFA procedures.
There is still debate about the number of ablations patients with squamous dysplasia should undergo. It is also not clear whether these should be performed at successive treatment encounters or whether these patients should undergo staging with chromoendoscopy after successive treatments. In our series, the median number of ablations was only one (range 1-3) for the 10 patients who achieved CR-D at 12 mo. These were all circumferential balloon ablations. This compares to a median of 3 ablations administered in patients with BE during a similar treatment protocol in published series. This may account for the lower eradication rates but the protocol was designed to provide an extremely cautious approach to restaging these patients after every treatment in view of the aggressive nature of the disease.
The rate of stricturing in our series was 20%. All 4 patients who developed strictures had undergone circumferential balloon ablation with the HALO 360 device. This rate is higher that noted in trials of ablation for Barrett’s oesophagus[27,28]. Nonetheless they were all overcome with straightforward dilatations. In a recent series of patients treated with RFA for squamous dysplasia the rate of stricturing was 14% after circumferential ablation[33]. Other than a self-limiting bleed from biopsies and not from a RFA procedure our study confirms that RFA is a safe procedure with few other reported adverse events.
A criticism of the protocol in the United Kingdom registry is that there may be too long an interval between treatments due to the requirement of a mapping endoscopy every 3 mo after RFA. With the aggressive nature of this disease it is very important to restage these patients early after treatment and perhaps shortening the intervals between treatment and follow up may help improve outcomes. Current United Kingdom practice is to only ablate once at each treatment session for patients with squamous neoplasia compared to the double ablation carried out in BE with mucosal cleaning of the coagulum between treatments. Recent publications have used 2 ablations per session safely in patients with squamous dysplasia and our practice may have to change to improve outcomes. Delivering 2 ablations at a single treatment session is standard practice for patients undergoing RFA for BE. Bergman and colleagues[33] explored various ablation energy settings in patients with squamous dysplasia. These included 2 ablations at a single treatment session with and without coagulum clearance between ablations. In 16 cases where they employed a 12 J/cm2 - clean - 12 J/cm2 protocol CR-D at 12 mo was 100% with a stricture rate of 19% compared to a CR-D of 86% and stricture rate of 14% with a 12 J/cm2 - no clean - 12 J/cm2 ablation protocol. By using lower energy settings of 10 J/cm2 for the second ablation or using 10 J/cm2 for delivering both ablations they did not compromise CR-D (100%) but interestingly had no strictures. The numbers in each of these treatment groups were however low (4 and 2 cases respectively).
Another short coming of our study is the small size of the cohort. These lesions are rarely diagnosed at an early stage. With the availability of high definition endosocopy and growing experience of minimally invasive endotherapy as an alternative to surgical and oncological interventions, these numbers will undoubtedly grow in the coming years.
This study examines data from 8 different centers nationwide. Despite standardized protocols, the expertise and experience of each endoscopist will be very different and individual preference and clinical practice will differ from one center to another. These patients have all undergone endotherapy within the confines of demanding endoscopy service provision. These data represent real life outcomes of integrating novel and ground breaking endotherapy to existing practice.
This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Oesophageal cancer carries a poor prognosis as it is often diagnosed at a stage where curative therapy is no longer possible. Squamous cell cancer (SCC) of the oesophagus carries a 5-year survival of 10%-15%. The precursor lesion to SCC is squamous dysplasia. Treatment of these early lesions with endo-luminal therapy may help to improve outcomes in these high risk patients.
Radiofrequency ablation (RFA) is a novel and minimally invasive field ablation technique that has shown good safety and efficacy for treating patients with Barrett’s related neoplasia in the oesophagus. By combining endoscopic mucosal resection for visible lesions and RFA, patients with early squamous neoplasia of the oesophagus may be treated at an early stage of the disease.
There are only limited series reporting the use of RFA in patients with early squamous neoplasia to date. These data better inform us on patients that are likely to succeed with endoscopic therapy but also the importance of careful staging in these patients before treatment.
This prospective study may better inform clinicians about minimally invasive endoscopic therapy in these patients where perhaps more radical treatments are not an option for patients.
The authors present a good study to evaluate the role of radiofrequency ablation in early squamous cell cancer and high grade squamous dysplasia. They provide a new finding of early response to RFA is prognostically important. This could be clinically relevant for physicians to perform RFA in the management of squamous high-grade dysplasia.
P- Reviewers Kumar A, Kang JC, Yen HH S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Blot WJ. Esophageal cancer trends and risk factors. Semin Oncol. 1994;21:403-410. [PubMed] [Cited in This Article: ] |
| 2. | Messmann H. Squamous cell cancer of the oesophagus. Best Pract Res Clin Gastroenterol. 2001;15:249-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, Schatzkin A. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424-1433. [PubMed] [Cited in This Article: ] |
| 4. | Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9. [PubMed] [Cited in This Article: ] |
| 5. | Yong EL, Han XP, Watson DI, Devitt PG, Jamieson GG, Thompson SK. Outcome following surgery for squamous cell carcinoma of the oesophagus. ANZ J Surg. 2009;79:724-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Jass JR, Sobin LH, Watanabe H. The World Health Organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162-2167. [PubMed] [Cited in This Article: ] |
| 7. | Dawsey SM, Lewin KJ, Wang GQ, Liu FS, Nieberg RK, Yu Y, Li JY, Blot WJ, Li B, Taylor PR. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686-1692. [PubMed] [Cited in This Article: ] |
| 8. | Matsubara T, Ueda M, Abe T, Akimori T, Kokudo N, Takahashi T. Unique distribution patterns of metastatic lymph nodes in patients with superficial carcinoma of the thoracic oesophagus. Br J Surg. 1999;86:669-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Araki K, Ohno S, Egashira A, Saeki H, Kawaguchi H, Sugimachi K. Pathologic features of superficial esophageal squamous cell carcinoma with lymph node and distal metastasis. Cancer. 2002;94:570-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Watanabe M, Kuwano H, Araki K, Kawaguchi H, Saeki H, Kitamura K, Ohno S, Sugimachi K. Prognostic factors in patients with submucosal carcinoma of the oesophagus. Br J Cancer. 2000;83:609-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Tajima Y, Nakanishi Y, Tachimori Y, Kato H, Watanabe H, Yamaguchi H, Yoshimura K, Kusano M, Shimoda T. Significance of involvement by squamous cell carcinoma of the ducts of esophageal submucosal glands. Analysis of 201 surgically resected superficial squamous cell carcinomas. Cancer. 2000;89:248-254. [PubMed] [Cited in This Article: ] |
| 12. | Tajima Y, Nakanishi Y, Ochiai A, Tachimori Y, Kato H, Watanabe H, Yamaguchi H, Yoshimura K, Kusano M, Shimoda T. Histopathologic findings predicting lymph node metastasis and prognosis of patients with superficial esophageal carcinoma: analysis of 240 surgically resected tumors. Cancer. 2000;88:1285-1293. [PubMed] [Cited in This Article: ] |
| 13. | Sgourakis G, Gockel I, Lyros O, Hansen T, Mildenberger P, Lang H. Detection of lymph node metastases in esophageal cancer. Expert Rev Anticancer Ther. 2011;11:601-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Sgourakis G, Gockel I, Lyros O, Lanitis S, Dedemadi G, Polotzek U, Karaliotas C, Lang H. The use of neural networks in identifying risk factors for lymph node metastasis and recommending management of t1b esophageal cancer. Am Surg. 2012;78:195-206. [PubMed] [Cited in This Article: ] |
| 15. | Pech O, Gossner L, May A, Vieth M, Stolte M, Ell C. Endoscopic resection of superficial esophageal squamous-cell carcinomas: Western experience. Am J Gastroenterol. 2004;99:1226-1232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Katada C, Muto M, Momma K, Arima M, Tajiri H, Kanamaru C, Ooyanagi H, Endo H, Michida T, Hasuike N. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy. 2007;39:779-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Tanaka T, Matono S, Nagano T, Murata K, Sueyoshi S, Yamana H, Shirouzu K, Fujita H. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc. 2011;73:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [PubMed] [Cited in This Article: ] |
| 20. | Ishihara R, Iishi H, Takeuchi Y, Kato M, Yamamoto S, Yamamoto S, Masuda E, Tatsumi K, Higashino K, Uedo N. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc. 2008;67:799-804. [PubMed] [Cited in This Article: ] |
| 21. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 22. | Nonaka K, Arai S, Ishikawa K, Nakao M, Nakai Y, Togawa O, Nagata K, Shimizu M, Sasaki Y, Kita H. Short term results of endoscopic submucosal dissection in superficial esophageal squamous cell neoplasms. World J Gastrointest Endosc. 2010;2:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Tamiya Y, Nakahara K, Kominato K, Serikawa O, Watanabe Y, Tateishi H, Takedatsu H, Toyonaga A, Sata M. Pneumomediastinum is a frequent but minor complication during esophageal endoscopic submucosal dissection. Endoscopy. 2010;42:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-264, 264.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 25. | Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 914] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 27. | Sharma VK, Jae Kim H, Das A, Wells CD, Nguyen CC, Fleischer DE. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol. 2009;104:310-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | van Vilsteren FG, Alvarez Herrero L, Pouw RE, ten Kate FJ, Visser M, Seldenrijk CA, van Berge Henegouwen MI, Weusten BL, Bergman JJ. Radiofrequency ablation for the endoscopic eradication of esophageal squamous high grade intraepithelial neoplasia and mucosal squamous cell carcinoma. Endoscopy. 2011;43:282-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Zhang YM, Bergman JJ, Weusten B, Dawsey SM, Fleischer DE, Lu N, He S, Wang GQ. Radiofrequency ablation for early esophageal squamous cell neoplasia. Endoscopy. 2010;42:327-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [PubMed] [Cited in This Article: ] |
| 31. | Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566-573; discussion 573-575. [PubMed] [Cited in This Article: ] |
| 32. | Pouw RE, Gondrie JJ, Curvers WL, Sondermeijer CM, Ten Kate FJ, Bergman JJ. Successful balloon-based radiofrequency ablation of a widespread early squamous cell carcinoma and high-grade dysplasia of the esophagus: a case report. Gastrointest Endosc. 2008;68:537-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Bergman JJ, Zhang YM, He S, Weusten B, Xue L, Fleischer DE, Lu N, Dawsey SM, Wang GQ. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc. 2011;74:1181-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Dawsey SM, Lewin KJ, Liu FS, Wang GQ, Shen Q. Esophageal morphology from Linxian, China. Squamous histologic findings in 754 patients. Cancer. 1994;73:2027-2037. [PubMed] [Cited in This Article: ] |
| 35. | Pech O, May A, Gossner L, Rabenstein T, Manner H, Huijsmans J, Vieth M, Stolte M, Berres M, Ell C. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoléon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, Miah H, Smart HL, Bhandari P, Smith LA. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145:87-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |