Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.17163
Revised: May 20, 2014
Accepted: July 15, 2014
Published online: December 7, 2014
AIM: To investigate the feasibility of separation and cultivation of circulating tumor cells (CTCs) in pancreatic cancer (PaC) using a filtration device.
METHODS: In total, 24 PaC patients who were candidates for surgical treatment were enrolled into the study. Peripheral blood samples were collected before an indicated surgery. For each patient, approximately 8 mL of venous blood was drawn from the antecubital veins. A new size-based separation MetaCell® technology was used for enrichment and cultivation of CTCs in vitro. (Separated CTCs were cultured on a membrane in FBS enriched RPMI media and observed by inverted microscope. The cultured cells were analyzed by means of histochemistry and immunohistochemistry using the specific antibodies to identify the cell origin.
RESULTS: CTCs were detected in 16 patients (66.7%) of the 24 evaluable patients. The CTC positivity did not reflect the disease stage, tumor size, or lymph node involvement. The same percentage of CTC positivity was observed in the metastatic and non-metastatic patients (66.7% vs 66.7%). We report a successful isolation of CTCs in PaC patients capturing proliferating cells. The cells were captured by a capillary action driven size-based filtration approach that enabled cells cultures from the viable CTCs to be unaffected by any antibodies or lysing solutions. The captured cancer cells displayed plasticity which enabled some cells to invade the separating membrane. Further, the cancer cells in the “bottom fraction”, may represent a more invasive CTC-fraction. The CTCs were cultured in vitro for further downstream applications.
CONCLUSION: The presented size-based filtration method enables culture of CTCs in vitro for possible downstream applications.
Core tip: Circulating tumor cells role in the process of pancreatic cancer dissemination should be studied in the context of the disease management. The ability to in vitro culture pancreatic circulating tumor cells (CTCs) could potentially help with the development of innovative treatments and diagnostic technologies. We presented simple size-based separation device for the isolation of viable CTCs. The isolation process is gentle allowing the subsequent CTC-cultivation in vitro and is antibody independent.
- Citation: Bobek V, Gurlich R, Eliasova P, Kolostova K. Circulating tumor cells in pancreatic cancer patients: Enrichment and cultivation. World J Gastroenterol 2014; 20(45): 17163-17170
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/17163.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.17163
The lethal nature of cancer is caused by its invasive character, and spread via blood and lymphatic system to distant locations generating metastatic disease. Moreover, pancreatic cancer (PaC) counts to the solid tumors with the shortest overall survivals. The aggressiveness of the disease is demonstrated in clinic by very early metastatic disease and chemoresistance[1-3].
Prognostic value of tumor cells disseminated to the blood and bone marrow has been shown for various types of solid tumors. Circulating tumor cells (CTCs) are cells shed from primary tumor and metastatic sites to the peripheral blood.
Large patient series of breast, prostate, lung, colon cancer have been tested for CTCs, but no complete results have been reported so far in pancreatic cancer clinical trials[4-6].
The limitation of the recently available tumor markers in PaC could be overcome by CTC.
The analytical methods developed to identify CTCs in PaC include direct and indirect CTCs- detection. Analytical assays based on antibodies against EpCAM antigen expressed on the cells surface count to the direct CTC- isolation methods together with size based separations. The polymerase chain reaction-based assays analyzing DNA and RNA count for indirect detection methods[7,8].
Characterization of CTCs in PaC including enumeration could be an important part of the diagnostic process. CTCs detection aims to reveal the tumor recurrence risk, chemo and radiotherapy resistance markers[9].
Moreover, the conventional prognostic indicators to predict patient outcome are often imperfect, owing mainly to tumor plasticity and subjective assessment criteria. Therefore, there is an urgent need for the establishment of new sensitive prognostic methods capable of identifying patients with a worse prognosis or those who will progress quickly.
In the present study, we have employed size-based separation method to detect CTCs. Our goal was to create an accurate assay that would improve the both detection and cultivation of CTCs/disseminated tumor cells (DTCs) of pancreatic cancer patients avoiding false-positive results and to allow for the personalization of therapy regimens.
To date, 24 patients with diagnosed PaC have been enrolled into the study in accordance with Declaration of Helsinki. All patients were candidates for surgery treatment, but 9 out of the 24 patients (37.5%) were seen as inoperable within surgery. Based on the informed consent clinical data were collected from all participating patients. The patient sample characteristics are shown in Table 1. Peripheral blood (PB) was collected prior to surgery. For each patient, peripheral blood (8 mL) was withdrawn into S-Monovette tubes (Sarstedt AG and Co., Numbrecht, Germany) containing 1.6 mg EDTA/mL blood as an anticoagulant. The isolation procedure was completed within 24 h after the blood withdrawal (the samples were stored at 4-8 °C up to 24 h).
| Patient characteristics | Total patients (n) | With detected CTC |
| T stage | 24 | |
| T1 | 0 | 0 (0) |
| T2 | 2 | 1 (50) |
| T3 | 11 | 9 (81.8) |
| T4 | 11 | 6 (54.5) |
| N stage | ||
| N0 | 7 | 6 (85.7) |
| N1 | 17 | 10 (58.9) |
| M stage | ||
| M0 | 21 | 14 (66.7) |
| M1 | 3 | 2 (66.7) |
| Grading | ||
| 1 | 1 | 0 (0) |
| 2 | 13 | 8 (61.5) |
| 3 | 9 | 8 (88.9) |
| 4 | 1 | 0 (0) |
| Disease stage | ||
| I | 1 | 1 (100) |
| IIA | 5 | 4 (80) |
| IIB | 7 | 5 (71.4) |
| III | 8 | 4 (50) |
| IV | 3 | 2 (66.7) |
Recently, a new size based separation method for viable CTC - enrichment from PB has been introduced (MetaCell®, MetaCell s.r.o., Ostrava, Czech Republic)[10]. The size-based enrichment process is based on the filtration of peripheral blood through a porous polycarbonate membrane (pores with 8 μm diameter). The minimum and maximum volume of the filtered PB may be adjusted up to 50 mL with fluid. The standard 8 mL of PB from patients suffering with PaC was transferred into the filtration tube-set. Successive blood transfer in several steps is preferred to prevent the blood clotting on the membrane filter. The PB flow is driven by capillary action of the absorbent touching the membrane filter. The whole isolation procedure runs at room temperature. The filtered CTCs were observed on the membrane immediately after filtration by light microscopy and subsequently (after 2 h) by fluorescent microscopy using unspecific nuclear stain (NucBlue™, Life Technologies). For some of the tested samples unspecific cytoplasmic stain (CellTracker™, Life Technologies) has been used as well to identify viable CTCs. The fluorescent analysis enables to distinguish cytomorphology of the recently enriched CTC fraction with a very high percentage of sensitivity.
The control of presence of the captured CTCs immediately after the isolation process helps to avoid false negative results of examination.
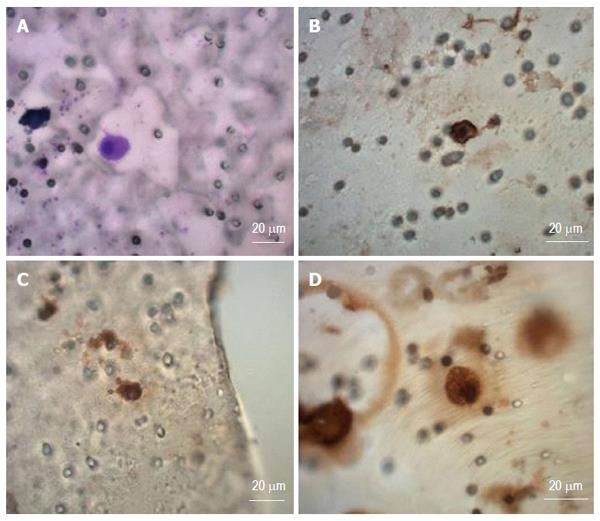
The membrane filter is kept in a plastic ring that is transferred into the 6-well cultivation plate, 2 mL RPMI media is added to the filter top and 2 mL to the well bottom. CTCs are cultured on the membrane in vitro under standard cell culture conditions (37 °C, 5% atmosphere of CO2) and observed by inverted microscope (Figure 1). The CTCs were grown in FBS enriched RPMI medium (10%) for the period of minimum 14 d on the membrane. The cultured cells were analyzed by means of histochemistry (May-Grünwald staining) and immunohistochemistry using the specific antibodies to identify cell origin [anti-cytokeratin 18 -FITC conjugated antibody (Sigma, Germany)], monoclonal CEA, cytokeratin CK7 and vimentin (Dako Denmark S/V) and unspecific DAPI staining (Sigma, Germany) (Figures 1, 2 and 3).
Alternatively the enriched CTCs fraction can be transferred from the membrane and cultured directly on any plastic surface or a microscopic slide. Microscopic slide culture is preferred if immunohistochemistry/immunofluorescence analysis is planned. If an intermediate CTCs-analysis is awaited, the CTCs-fraction is transferred in PBS (1.5 mL) to a cytospin slide. The slide is then dried for 24 h and analyzed by means of immunohistochemistry.
The fixed and stained cells on the membrane were examined using light and fluorescent microscopy. The analytical process can be divided into two steps: (1) observing at smaller magnification (up to × 20) to identify cells or cell nuclei; and (2) observing at higher magnification (up to × 6) for more detailed evaluation of cytomorphology.
Cells captured on the separating membrane (single cells or cells within clusters) were located, digitized, and evaluated by a trained researcher and/or experienced pathologist. Cells presenting below listed characteristics were defined as CTCs: (1) nuclear size equal or larger than 10 μm); (2) presence of a visible cytoplasm; (3) prominent nucleoli; (4) high nuclear-cytoplasmic ratio, which is not necessarily true in case of in vitro cultured cells; and (5) irregular nuclear contour.
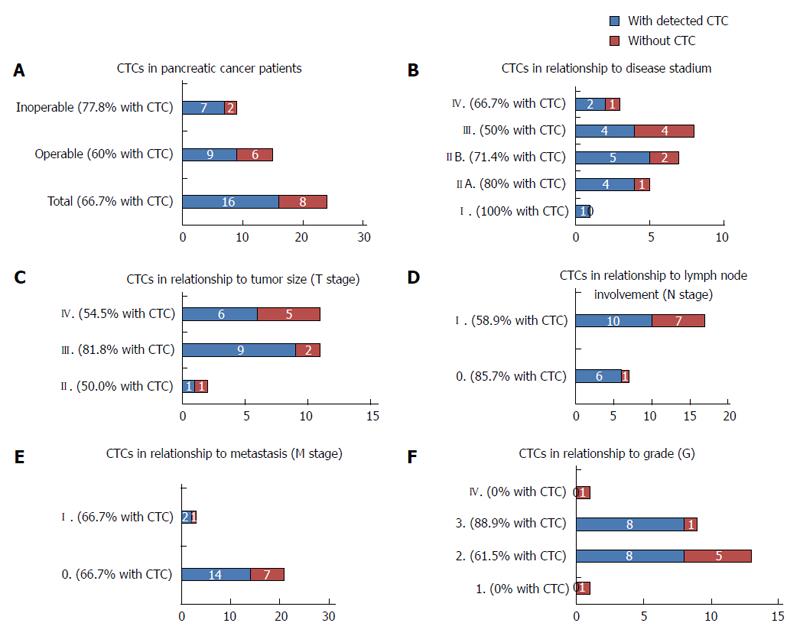
The frequency of the CTCs positivity is summarized for different patient subgroups in Figure 4. CTCs were detected in 16 patients of 24 patients (66.7 %), (Figure 4A), with comparable frequencies in operable and inoperable patients (60% vs 77.8%). Due to the low number of patients in the different disease stage groups, CTC-positivity does not reflect the disease stage (Figure 4B), tumor size (Figure 4C), or lymph node involvement (Figure 4D). The same percentage of CTC positivity was observed in the metastatic and nonmetastatic patients (66.7% vs 66.7%) (Figure 4E). Interestingly 88.9% patients with tumor grade 3 were affected by the spread of the pancreatic disease defined by CTCs (Figure 4F). The CTC - positivity reported for each patient individually is shown in the Table 2. We are not able to report any correlation of CTC-abundance and histology tumor subtype.
| Stadium | Tumor histology | CTC | T | N | M | G | Surgery |
| IB | Ductal | YES | 2 | 0 | 0 | 2 | Operabile |
| IIA | Cylindrocellular | YES | 3 | 0 | 0 | 3 | Operabile |
| IIA | Ductal | YES | 3 | 0 | 0 | 3 | Operabile |
| IIA | Ductal | YES | 3 | 0 | 0 | 3 | Operabile |
| IIA | Ductal | NO | 3 | 0 | 0 | 2 | Inoperabile |
| IIA | Ductal | YES | 3 | 0 | 0 | 2 | Operabile |
| IIB | X | YES | 3 | 1 | 0 | 3 | Inoperabile |
| IIB | Ductal | YES | 3 | 1 | 0 | 3 | Inoperabile |
| IIB | X | NO | 3 | 1 | 0 | 2 | Inoperabile |
| IIB | Ductal | YES | 3 | 1 | 0 | 3 | Operabile |
| IIB | Ductal | YES | 3 | 1 | 0 | 2 | Operabile |
| IIB | Ductal | YES | 3 | 1 | 0 | 2 | Inoperabile |
| IIB | Anaplastic | NO | 2 | 1 | 0 | 4 | Operabile |
| III | Ductal | NO | 4 | 1 | 0 | 2 | Operabile |
| III | Ductal | NO | 4 | 1 | 0 | 2 | Operabile |
| III | Ductal/cylindrocellular | NO | 4 | 1 | 0 | 1 | Operabile |
| III | Ductal | YES | 4 | 1 | 0 | 3 | Inoperabile |
| III | Ductal/cribriform | NO | 4 | 1 | 0 | 2 | Operabile |
| III | Ductal | YES | 4 | 0 | 0 | 2 | Operabile |
| III | Ductal/neuroendocrine | YES | 4 | 1 | 0 | 2 | Inoperabile |
| III | Ductal | YES | 4 | 1 | 0 | 3 | Inoperabile |
| IV | Ductal | YES | 4 | 1 | 1 | 2 | Inoperabile |
| IV | Ductal | NO | 4 | 1 | 1 | 3 | Operabile |
| IV | Ductal | YES | 4 | 1 | 1 | 2 | Operabile |
We evidence successful CTCs isolation in patients with pancreatic cancer, capturing viable cells with proliferation potential. The cells captured by size-based filtration approach are enriched with good fitness, what enables the culture of the CTCs unaffected by any antibodies or lysing solutions. The CTCs were cultured in vitro for further downstream applications. The confluent cell growth was reached in the majority of the cultivated CTC cases.
The size of the captured cells guided us in the cancer cell identification process even without any additional staining (e.g., May-Grűnwald -MGG). This standard staining protocol (MGG) has enabled us to analyze the nuclei including nucleoli. Generally the nucleus was bigger than 10 μm itself and the cells did not present much of cytoplasm immediately after separation process. The nuclear-cytoplasmatic ratio is relatively high in cancer cells, but not in the in vitro cultured CTCs. The CTCs get big and long in the culture, changing the nuclear-cytoplasmatic ratio. The cytoplasm of CTCs is rather pale than condensed.
Due to the cell size (15 μm), nucleus size (10 μm),shape, and nucleoli visualized by MGG or simple DAPI-stain in the formerly fixed cells, cancer cells are detected on the separating membrane (Figure1A), and also on the plastic bottom of the 6-well plate (Figure 1B-D). These results indicate that the captured cancer cells display plasticity enabling to grow through the separating membrane. Concerning the shape of the cancer cells in the “bottom fraction”, these cells could present a more invasive CTC-fraction with a spindle cell-like shape. The immunohistochemical analysis has shown the abundance of the cytokeratin-18 in the “membrane” fraction as well as in the “bottom” fraction proving the carcinoma origin. We see an enormous potential in the gene expression analysis, which could reveal the epithelial - mesenchymal character of the detected cancer cells. The mesenchymal like type phenotype of CTCs after an epithelial- mesenchymal transition (EMT), was described by expression of CK7, CEA and vimentin (Figure 3).
The presented study aimed to successful isolation of CTCs from patients with PaC using a simple size-based separation device. The gentle antibody independent isolation process allows the subsequent CTC-cultivation in vitro.
The antibody independence could be of advantage because the detection of CTCs cannot be based only on the expression of epithelial makers (EpCAM) or cytokeratins due to their lost within EMT process[11-13].
The epithelial markers can be down regulated during tumor cell dissemination, affecting the detection rates of CTCs[14-16]. Relatively low CTC-detection rates were reported using the isolation methods relying on EpCAM expression (e.g., CellSearch®, Adnagen®) in the early disease stages suggesting, EpCAM-based isolation processes may not be efficient for CTC-detection[17-19].
Two platforms for CTC- enumeration based on different isolation principles (CellSearch® and ISET) were compared in the study of pancreatic cancer by Khoja et al[20] prospectively. CellSearch® works on the antibody dependent principle of CTCs-separation. All CTCs expressing epithelial markers should be captured by immunomagnetic separation. ISET is asize-based, blood filtration device. It has been reported that CTCs were detected in 93% of patients via ISET and in 40 % by CellSearch®.
We detected CTCs in the 66.7% cases. If the patients are subcategorized, the CTC- positivity reaches 80% in some subgroups (e.g., T3-stage). In discussing the results of the CTC-frequency in the pre-defined patient subgroups we should report the relationship between the CTCs and operability of the PaC patients. The CTCs were detected in the 77.8 % of inoperable patients. In the future the CTCs-tests could be used for pre-screening patients before operation. Stage III encompasses patients whose tumors can be surgically removed which represents more than a half of the patients. The CTCs positivity results show, that also in the resectable cases, the CTC-positivity number is very high (60%). There is a possibility to pre-treat the CTC-positive patients within neoadjuvant therapy (chemotherapy or radiotherapy) combined regimens. The survival analysis of the PaC patients in the tested group is not available yet to show how different the patient performance and therapy resulted following CTC testing.
We have to acknowledge the potential of CTCs in understanding and discovering the biological principles of cancer dissemination. The information on CTCs should help to manage patients according the CTC- presence. The in vitro proliferation of CTCs in PaC could enable an implementation of new analytical methodologies such as genome profiling, microRNA studies, protein expression testing, chemoresistance analysis, and discovery of new therapeutic regimens and targets. To use the CTCs as biomarker monitoring the therapeutic efficiency in PaC could be of advantage in near future. Subsequently, CTC-testing is helping to improve patient stratification for new targeted drugs regimens[21-25].
Nevertheless, due to the lack of treatment possibilities in PaC, it is not possible yet to identify by help of CTCs new “druggable targets”.But it is possible to identify in CTCs susceptible genes, which could be used for gene therapy development and immunotherapy optimalization in individual patients[7].
CTCs may help to identify differences between primary tumor and metastasis on the phenotype and genotype level. The clonal heterogeneity of the primary tumor could explain differences between populations of disseminated cells and the evolution of their new genotypes and phenotypes[26]. To identify the differences on the gene expression level between the primary tumor cells and CTCs should be a base for improving the therapeutical approaches. To answer all the questions discussed above one needs larger number of CTCs. There are several ways how to obtain larger numbers of cells: (1) have more precise and specific separation techniques; (2) To analyze bigger blood volumes; or (3) culture CTCs in vitro enabling their proliferation and further downstream analysis. This could be reached by separation technologies as presented here. The newly reported successful in vitro CTCs-culture could be a tool to overcome the low-number CTCs limits, enabling any further research e.g., on protein expression level.
Metastasis in visceral tissue is the major cause of cancer death .The dissemination process is based on the plasticity of malignant cells enabling their migration from primary tumors to other microenvironments. Hematogenous dissemination is the most common dissemination route even in tumors spreading through lymphatic system. Circulating tumor cells (CTCs) are messengers in the cancer dissemination process-holding a promise to determine new therapeutical regimens for the advanced cancer. There have been many clinical studies which showed the utility of CTCs abundance in the bloodstream as a prediction and prognostic marker of the pancreatic disease.
The authors still don’t know yet how many tumor cells and how often are shed from the primary tumor into the bloodstream. It is hypothesized that 1 g of tumor tissue may release 106 of the cells into to bloodstream every 24 h. It is essential to establish sensitive and specific technologies to detect CTCs, which would enable their analysis on molecular level to further understand CTCs biology. CTCs present an opportunity to offer information on more effective treatment strategies and metastatic process prevention in individuals.
Innovation and improving of CTC detection methods has been reported recently for CTC microchips, filtration based methodologies, quantitative reverse transcription polymerase chain reaction. Improvements in CTC capture efficiency, quantification, imaging and molecular analyses are likely to enable further clinical applications. In this original research paper, we demonstrate that it is possible to isolate human CTCs from patients with pancreatic cancer, with subsequent cultivation and proliferation in vitro.
CTCs provide a novel prognostic and predictive biomarker enabling to monitor the efficacy of systemic therapy. CTCs may help to identify differences between primary tumor and metastasis on the phenotype and genotype level. The clonal heterogeneity of the primary tumor could explain differences between populations of disseminated cells and the evolution of their new genotypes and phenotypes. To identify the differences on the gene expression level between the primary tumor cells and CTCs, CTCs should be a base for improving the therapeutical approaches.
CTCs the cancer cells circulating in the bloodstream. Size-based enrichment process: CTC-enrichment based on cell size, addresses the problem of reduced EpCAM expression.
This paper provides a size-based method to isolate and culture pancreatic CTCs from clinical blood samples. The major point of this paper is the culture of CTCs. Many downstream tests are possible for the characterizations of these cells.
P- Reviewer: Huang THM, Rigaud M S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8406] [Cited by in F6Publishing: 8901] [Article Influence: 741.8] [Reference Citation Analysis (0)] |
| 2. | De La Cruz MS, Young AP, Ruffin MT. Diagnosis and management of pancreatic cancer. Am Fam Physician. 2014;89:626-632. [PubMed] [Cited in This Article: ] |
| 3. | Hackert T, Büchler MW. Pancreatic cancer: advances in treatment, results and limitations. Dig Dis. 2013;31:51-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Liberko M, Kolostova K, Bobek V. Essentials of circulating tumor cells for clinical research and practice. Crit Rev Oncol Hematol. 2013;88:338-356. [PubMed] [Cited in This Article: ] |
| 5. | Eliasova P, Kolostova K, Kobierzycki C, Bobek V. Clinical studies monitoring circulating and disseminated tumor cells in gastrointestinal cancers. Folia Histochem Cytobiol. 2013;51:265-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kaur S, Baine MJ, Jain M, Sasson AR, Batra SK. Early diagnosis of pancreatic cancer: challenges and new developments. Biomark Med. 2012;6:597-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Cen P, Ni X, Yang J, Graham DY, Li M. Circulating tumor cells in the diagnosis and management of pancreatic cancer. Biochim Biophys Acta. 2012;1826:350-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Mikulová V, Kološtová K, Zima T. Methods for detection of circulating tumour cells and their clinical value in cancer patients. Folia Biol (Praha). 2011;57:151-161. [PubMed] [Cited in This Article: ] |
| 9. | Ren C, Han C, Zhang J, He P, Wang D, Wang B, Zhao P, Zhao X. Detection of apoptotic circulating tumor cells in advanced pancreatic cancer following 5-fluorouracil chemotherapy. Cancer Biol Ther. 2011;12:700-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Bobek V, Kacprzak G, Rzechonek A, Kolostova K. Detection and cultivation of circulating tumor cells in malignant pleural mesothelioma. Anticancer Res. 2014;34:2565-2569. [PubMed] [Cited in This Article: ] |
| 11. | Raimondi C, Gradilone A, Naso G, Vincenzi B, Petracca A, Nicolazzo C, Palazzo A, Saltarelli R, Spremberg F, Cortesi E. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011;130:449-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Peeters DJ, van Dam PJ, Van den Eynden GG, Rutten A, Wuyts H, Pouillon L, Peeters M, Pauwels P, Van Laere SJ, van Dam PA. Detection and prognostic significance of circulating tumour cells in patients with metastatic breast cancer according to immunohistochemical subtypes. Br J Cancer. 2014;110:375-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Fong D, Moser P, Kasal A, Seeber A, Gastl G, Martowicz A, Wurm M, Mian C, Obrist P, Mazzoleni G. Loss of membranous expression of the intracellular domain of EpCAM is a frequent event and predicts poor survival in patients with pancreatic cancer. Histopathology. 2014;64:683-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Lowes LE, Allan AL. Recent advances in the molecular characterization of circulating tumor cells. Cancers (Basel). 2014;6:595-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013;73:8-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Sterzynska K, Kempisty B, Zawierucha P, Zabel M. Analysis of the specificity and selectivity of anti-EpCAM antibodies in breast cancer cell lines. Folia Histochem Cytobiol. 2012;50:534-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Hofman V, Ilie MI, Long E, Selva E, Bonnetaud C, Molina T, Vénissac N, Mouroux J, Vielh P, Hofman P. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129:1651-1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Sandri MT, Zorzino L, Cassatella MC, Bassi F, Luini A, Casadio C, Botteri E, Rotmensz N, Adamoli L, Nolè F. Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol. 2010;17:1539-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Khoja L, Backen A, Sloane R, Menasce L, Ryder D, Krebs M, Board R, Clack G, Hughes A, Blackhall F. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Gall TM, Frampton AE, Krell J, Jacob J, Stebbing J, Jiao LR. Is the detection of circulating tumor cells in locally advanced pancreatic cancer a useful prognostic marker? Expert Rev Mol Diagn. 2013;13:793-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Tjensvoll K, Nordgård O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer. 2014;134:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 97] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, Liu J, Pantel K. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18:5701-5710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3360] [Cited by in F6Publishing: 3257] [Article Influence: 162.9] [Reference Citation Analysis (0)] |
| 25. | Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. 2014;51:160-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Han L, Chen W, Zhao Q. Prognostic value of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35:2473-2480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |