Article Text
Abstract
Backround and aims: The aetiopathogenesis of Crohn’s disease, an inflammatory bowel disease (IBD), is not yet fully understood. Autoimmune mechanisms are thought to play a role in the development of Crohn’s disease, but the target antigens and the underlying pathways have not been sufficiently identified.
Methods: Based on data from immunoblotting and matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) mass spectrometry, the major antigenic target of pancreatic autoantibodies (PABs), which are specific for Crohn’s disease, was identified. Specificity of autoantibody reactivity was confirmed by enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence (IIF) using purified rat and human recombinant GP2 synthesised in transiently transfected mammalian HEK 293 cells. Real-time polymerase chain reaction (rt-PCR) and IIF were used to detect mRNA and antigen localisation in human colon biopsies.
Results: The major zymogen granule membrane glycoprotein 2 (GP2) was identified as the autoantigen of PABs in Crohn’s disease. PAB-positive sera from patients with Crohn’s disease (n = 42) displayed significantly higher IgG reactivity to rat GP2 in ELISA than either PAB-negative sera (n = 31), or sera from patients with ulcerative colitis (n = 49), or sera from blood donors (n = 69) (p<0.0001, respectively). Twenty-eight (66%) and 18 (43%) of 42 PAB-positive sera demonstrated IgG and IgA reactivity to human recombinant GP2 in IIF, respectively. Patients with PAB-negative Crohn’s disease (n = 31) were not reactive. GP2 mRNA transcription was significantly higher in colon biopsies from patients with Crohn’s disease (n = 4) compared to patients with ulcerative colitis (n = 4) (p = 0.0286). Immunochemical staining confirmed GP2 expression in human colon biopsies from patients with Crohn’s disease.
Conclusion: Anti-GP2 autoantibodies constitute novel Crohn’s disease-specific markers, the quantification of which could significantly improve the serological diagnosis of IBD. The expression of GP2 in human enterocytes suggests an important role for anti-GP2 response in the pathogenesis of Crohn’s disease.
Statistics from Altmetric.com
Crohn’s disease and ulcerative colitis are the two most prevalent inflammatory bowel diseases (IBDs) in Caucasians in developed countries, affecting as many as one in 250 individuals. Nonetheless, the aetiologies of both disorders are not yet fully understood.1 Contrary to ulcerative colitis, the intestinal inflammation associated with Crohn’s disease is not confined to rectum and colon, but can affect any part of the gastrointestinal tract. The pathological feature of Crohn’s disease is a transmural inflammation of all layers of the bowel wall and adventitia. Therefore, tissue lesions such as fissures, abscesses, strictures and fistulas can occur.
Current evidence suggests that genetic predisposition and environmental factors play a crucial part in such inappropriate intestinal immune responses.1 Mucosal inflammation is presumed to develop when dysregulation of the immune system leads to an imbalance between tolerance to commensal microbiota or food-derived antigens and immunity to pathogens. Triggers of inflammation include impaired clearance of over-reactive or autoreactive T cell populations in combination with increased leakiness of the epithelial barrier, disturbance of innate epithelial immune mechanisms, disturbance of antigen recognition and processing of professional and atypical antigen-presenting cells, as well as disturbance of regulatory and effector T cell balance.2 3 4 5 6
Autoimmune mechanisms are also thought to play a role in the development of IBDs, but the target antigens and the underlying pathways have not been sufficiently characterised and identified.7 Autoantibody response in Crohn’s disease and ulcerative colitis is considered to be an epiphenomenon directed against antigens produced in exocrine pancreas, neutrophils and intestinal goblet cells.8 9
Patients with IBD have disorder-specific (auto)antibodies that may aid in the differential diagnosis of IBD, especially in the case of indeterminate colitis.10 11 Pancreatic autoantibodies have been detected by IIF in up to 31% of all patients with Crohn’s disease, and they occur in conjunction with disease-specific antibodies to bacterial peptides and glycans.8 9 10 11 12 13 14 (Auto)antibody-based disease prediction or stratification and correlation of (auto)antibody titres with disease activity or clinical symptoms remain controversial issues.11 However, antibodies against bacterial peptides and glycans have been shown to be associated with the severity of disease.12 Pathogenetically relevant IBD-specific (auto)antibodies have not been identified yet.
Several attempts to identify the target(s) of PABs in Crohn’s disease have failed.8 9 14 The presumed antigenic target has been described as a trypsin-sensitive macromolecular protein complex of more than 1000 kDa that is present in pancreatic juice.9
Here, we provide evidence that GP2, the major zymogen granule membrane glycoprotein, is the major autoantigen of PABs involved in Crohn’s disease. The relevance of this antibody–antigen system to the pathogenesis of Crohn’s disease is discussed in consideration of the homology of GP2 to Tamm–Horsfall protein (THP) of the urinary tract and the reported detection of GP2 in murine microfold or membranous cells (M cells) of Peyer’s patches.15 Furthermore, the expression of the targeted antigen GP2 was confirmed at the site of inflammation in Crohn’s disease.
Patients and methods
Patient sera were selected as previously described.10 Serum samples from 73 patients with Crohn’s disease and 49 patients with ulcerative colitis (supplied by the Pediatric Clinic, Technical University of Dresden and in.vent Diagnostica, Hennigsdorf, Germany) and 69 control sera from healthy blood donors were included in the study. All samples were taken at the time of consent and enrolment. The age of the 73 patients with Crohn’s disease (42 females, 31 males) ranged between 20 and 72 years (median, 41 years). The age of the 49 patients with ulcerative colitis (29 females, 20 males) ranged between 21 and 72 years (median, 40 years). The diagnoses of ulcerative colitis and Crohn’s disease were based upon standard clinical, radiological, endoscopic and histological criteria.16
Forty-two of the 73 patients with Crohn’s disease were identified as PAB-positive in IIF, whereas three out of 49 patients with ulcerative colitis and one out of 69 blood donor sera had positive PAB reactivity.
For analysis of intestinal GP2 expression, biopsy specimens were obtained consecutively during colonoscopy from four patients with Crohn’s disease (two females, two males; age 25–53 years), four patients with ulcerative colitis (two females, two males; age 26–50 years), and from five controls (three females, two males; age 24–72 years). In patients with IBD, colonoscopy was performed for routine diagnostic purposes. Controls underwent colonoscopy for cancer detection, herein macroscopic and histological analysis of the colon revealed no evidence of inflammatory bowel diseases. All patients and controls were recruited from the Department of Gastroenterology, Charité Berlin, Campus Mitte. Biopsies were taken from macroscopically involved (inflamed) areas. The decision of macroscopic involvement was made by the investigator (CB).
Animals and pancreatic tissue samples
Wistar rats (200–250 g) were obtained from the Animal Facility, Charité Hospital, Berlin, Germany. The animals were killed by asphyxiation with CO2, and the pancreas was excised, immediately frozen in liquid nitrogen, and stored at −80°C.
Two-dimensional electrophoresis and immunoblotting
Rat pancreas (300 mg) frozen in liquid nitrogen was crushed in a chilled mortar with a pestle, homogenised in 5 ml isoelectric focusing (IEF) sample buffer containing 9 mol/l urea, 2% ampholyte pH 7–9, 1 mmol/l diisopropylfluorophosphate (DFP), 50 mmol/l dithiothreitol (DTT), and subsequently clarified by centrifugation. The soluble pancreatic fraction was separated under denaturing conditions by two-dimensional gel electrophoresis employing the Mini-Vertical Gel electrophoresis Unit SE250 with SE220 Tube Gel Adaptor (Hoefer Pharmacia Biotech, San Francisco, California, USA) and 8 mol/l urea in the IEF step.17 The tube gel for IEF was 1.5 mm×70 mm and the flat gel for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was 60×80×1.5 mm.
Separated polypeptides were visualised using Coomassie Brilliant Blue G250 (Roth, Karlsruhe, Germany). Samples for immunoblotting were transferred to nitrocellulose membranes (Schleicher und Schüll, Dassel, Germany) by semi-dry blotting and blocked with 1% skimmed milk in phosphate-buffered saline and 0.1% Tween 20 (SM-PBS) at room temperature (RT) for 1 h. The membranes were then incubated with patient sera diluted 1 to 200 in SM-PBS for 1 h, subsequently washed with PBS, and incubated with horseradish peroxidase-conjugated anti-human immunoglobulin at RT for 30 min. Immunoreactive spots were visualised on photographic X-OMAT UV PLUS film (Kodak, Stuttgart, Germany) by enhanced chemiluminescence (ECL) (Roth).
Matrix-assisted laser desorption ionisation time of flight mass spectrometry
The molecular target of PAB response was identified using a 4700 Proteomics Analyser MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Foster City, California, USA). The protein within the specific reaction spot identified by ECL was digested in-gel by trypsin, and the peptides obtained in this manner were analysed by mass spectrometry. Using the resulting peptide mass fingerprint peak list together with an MS/MS peak list, we then searched the entire SwissProt 54.6 database using the Mascot MS/MS ion search engine in order to identify the target protein.17 The primary identification criterion was a significant (p<0.05) search result with a total score ⩾67.
Isolation of zymogen granules and purification of GP2
Zymogen granules (ZG) were isolated as described previously.18 Briefly, the pancreas of fasted male Wistar rats (200–250 g; Animal Facility, Charité Hospital) was excised. The pancreatic tissue was homogenised on ice in 0.3 mol/l sucrose, pH 6.8. The homogenate was filtered through gauze and centrifuged at 500 g for 10 min at 4°C to obtain the postnuclear supernatant. The supernatant was further centrifuged at 3000 g for 10 min at 4°C to pellet the zymogen granules. After removing the upper brownish layer (mainly mitochondria) by adding and decanting 10 mmol/l morpholinopropanesulfonic acid (MOPS) buffer (pH 6.8), zymogen granules were lysed in 0.1 mol/l sodium carbonate and 1 mmol/l DFP at 4°C for 1 h. Subsequently, the mixture was layered onto a step gradient of 0.3 and 1.0 mol/l sucrose and centrifuged at 200 000 g for 90 min. The membrane-enriched fraction was harvested and adjusted to 0.3 mol/l sodium bromide. After centrifugation at 200 000 g for 60 min, the membrane pellet was resuspended in 20 mmol/l cleavage buffer (morpholinoethanesulfonic acid (MES), 80 mmol/l potassium chloride, and 45 μg/ml saponin, pH 7.0). For solubilisation of GP2, 500 milliunits of phosphoinositol-specific phospholipase C (Bacillus cereus; Sigma-Aldrich, Taufkirchen, Germany) was added to the zymogen granule membrane fraction (corresponding to 1 mg protein), and the mixture was incubated at 37°C for 1 h. Membranes were pelleted by centrifugation at 200 000 g for 60 min. The GP2-containing supernatant was concentrated 5-fold by ultrafiltration and stored until further use at 4°C.
Antibody production
Polyclonal antibodies to GP2 were produced immunising rabbits with recombinant rat GP2 (donated by Dr Schrader, Gießen, Germany) according to a standard immunisation protocol (three cycles of injection of 100 μg protein). This was done at the Seramun Animal Facility (Seramun, Heidesee, Germany).
Enzyme-linked immunosorbent assay
Glycoprotein 2 autoantibodies in the patient sera were detected using an ELISA technique employing purified rat GP2 as solid-phase antigen. Briefly, 5 μg/ml GP2 was coated on Maxisorb microtitre plates (Nunc, Roskilde, Denmark) in bicarbonate buffer (pH 9.5) for 26 h at 4°C. After blocking with 0.05 mol/l Tris-HCl, 1% bovine serum albumin (Tris-BSA, pH 7.4) at RT for 1 h, serum samples diluted 1:100 in Tris-BSA were incubated at RT for 1 h and washed. Horseradish peroxidase-conjugated anti-human IgG or IgA (Seramun) was added and developed with ready-to-use H2O2/tetramethylbenzidine substrate (Seramun). The reaction was stopped with 0.25 mol/l sulfuric acid after 15 min. The optical density (OD) of the samples was read using a microplate reader (SLT, Crailsheim, Germany) at a wavelength of 450 nm/620 nm. Sera were considered positive when their OD exceeded the 95 percentile of OD values measured in the sera of healthy blood donors.
Indirect immunofluorescence assay on tissue sections
Antibodies to pancreas tissue were detected by running patient samples and the polyclonal anti-GP2 antibody on commercially available pancreas tissue sections according to the recommendations of the manufacturer (GA Generic Assays, Dahlewitz, Germany). Briefly, tissue sections were incubated in a moist chamber at RT for 30 min with 50 μl of serially diluted serum, starting at a concentration of 1:10. After washing, immune complexes were detected by incubating the samples with fluorescein-conjugated sheep anti-human IgG or IgA and anti-rabbit immunoglobulin (Seramun) for 30 min at RT. Samples were subsequently washed, embedded, and analysed by using a fluorescence microscope (Axiovert 40; Zeiss, Göttingen, Germany).
For the inhibition studies, PAB-positive human sera at the lowest dilution producing a positive reaction were pre-incubated with purified rat GP2 or bovine serum albumin (Sigma) at decreasing concentrations starting from 5 mg/l for 1 h at RT.
For GP2 detection in tissue sections of IBD and control patients, 5 μm paraffin sections of selected biopsies were probed with PABs as described above.
Indirect immunofluorescence assay on GP2 transfected mammalian cells
HEK293 cells and HEK293 cells transiently transfected with plasmid EX-MO474-M08-GP2 containing the full length human GP2 (GeneCopoeia, Germantown, Maryland, USA) by electroporation were purchased from CCS Cell Culture Service, Hamburg, Germany. Transfected and non-transfected cells as controls were cultured in Dulbecco’s modified Eagle’s medium (Biochrom, Berlin, Germany) containing 10% fetal bovine serum (Biochrom) and 1 mmol/l sodium pyruvate (Sigma), and 500 mg/l geneticin disulfate G418 (AppliChem, Darmstadt, Germany) on microscopy slides. After fixation with acetone (Sigma), patient samples diluted 1:40 and anti-GP2 rabbit serum were run on cells fixed on slides by IIF as described above.
RNA isolation from biopsies
To avoid degradation of RNA, biopsies were immediately placed in RNAlater (Quiagen, Hilden, Germany) and kept at −20°C until processing. RNA was isolated using the NucleoSpin RNA XS kit (Macherey–Nagel, Düren, Germany) according to the manufacturer’s instructions, including an on-column DNA digestion. Afterwards, RNA was reverse transcribed into cDNA using the Superscript III reverse transcriptase reagents from Invitrogen (Karlsruhe, Germany).
Primer design and real-time reverse-transcription polymerase chain reaction
Two primer pairs were selected based on a fragment of the GP2 transcript sequence for splice variant 1 (gene databank accession number NM_001007240) common for the other three splice variants of GP2 (splice variant 2 accession number NM_001502; splice variant 3 accession number NM_001007241; splice variant 4 accession number NM_001007242), ensuring detection of the four known GP2 variants. Primers were stringently designed with a length of 19–21 base pairs (bp) and an optimal annealing temperature of 62°C (SD 2°C) to amplify cDNA fragments between 180 and 210 bp, using the Primer 3 Software (Version 0.4.0; Whitehead Institute for Biomedical Research, Cambridge, Massachusetts, USA). The first primer pair (GP2-1, forward 5′-CAATGTGCCTACCCACTGGA-3′, reverse 5′-ATGGCACCCACATACAGCAC-3′) comprised a sequence of 200 bp between positions 411–610 of the transcript sequence, while the second primer pair (GP2-2, forward 5′-TCAACGTGATTCCACCATCC-3′, reverse 5′-TAGGTCGATGGCCGGTACTT-3′) amplified a 202 bp fragment between positions 728–929.
PCR amplification products were controlled by forward and reverse sequencing (DLMB, Rüdersdorf, Germany). Resulting sequences were analysed against gene databases using a BLAST similarity search and were then aligned using the ClustalW2 multiple sequence alignment (EMBL-EBI, Hinxton, Cambridge, UK).
Assessment of GP2 gene expression was performed by rt-PCR using the GeneAmp 5700 Sequence Detection System and the SYBR Green PCR Master Mix (PE Biosystems/PE Applied Biosystems, Foster City, California, USA) according to the manufacturer’s instructions and as described elsewhere.19 Co-amplification of non-specific products was controlled with a dissociation protocol after each PCR run. Expression of GP2 was quantified relative to the housekeeping genes β-actin (accession number BC_002409; forward primer 5′-CTGGACTTCGAGCAAGAGATG-3′, reverse primer 5′-TGAAGGTAGTTTCGTGGATGC-3′) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (accession number NM_002046, forward primer 5′-ATGTTCGTCATGGGTGTGAA-3′, reverse primer 5′-ACAGTCTTCTGGGTGGCAGT-3′).
For analysis of results, the amount of target gene GP2 was normalised to β-actin and GAPDH. Expression levels were calculated according to the formula 2− (ΔCt target − house keeping gene), where the threshold cycle (Ct) represents the fractional cycle number at which the amount of amplified target reaches a fixed threshold.20
Statistical analysis
A Kolmogorov–Smirnov test was used to test the data for normality. The measured values were expressed as medians with 95% confidential intervals (CIs). A two-tailed, non-parametric Mann–Whitney test for independent samples was used to test for statistically significant differences. p values of less than 0.05 were considered significant. Calculations were performed using Medcalc statistical software (Medcalc, Mariakerke, Belgium) and GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California, USA).
Results
Identification of GP2 as antigenic target of PABs
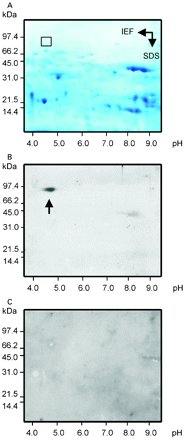
To identify the antigenic target of PABs, we first collected, aliquoted and stored five serum samples from patients with Crohn’s disease and with high titres of PABs (>1/320) at −20°C. Rat pancreatic tissue was homogenised, and the soluble fraction was subjected to two-dimensional gel electrophoresis (fig 1A). The separated pancreatic molecules were semi-dry blotted onto nitrocellulose membrane and tested with PAB-positive sera from patients with Crohn’s disease. To achieve the most sensitive detection level, bound human autoantibodies were visualised by ECL using anti-human IgG coupled to peroxidase (fig 1B). A specific reaction spot at an apparent molecular weight of 80 kDa and an apparent isoelectric point of pH 4.8 could be observed on the developed photographic films for four out of five high-titre PAB-positive sera investigated. Low-titre PAB-positive samples were not reactive. Areas of the gel corresponding to the specific reaction spot on the films were excised, and MALDI-TOF mass spectrometry was performed for molecule identification (fig 2).17 A database search was then performed to determine the molecular target of PAB response. Glycoprotein 2, the major glycoprotein associated with zymogen granule membranes in the pancreas, was identified with a scoring factor of 106, sequence coverage of 25%, and confirmation of one peptide by MS/MS.
Two-dimensional electrophoresis of the soluble fraction of homogenised rat pancreatic tissue. Pancreatic proteins were separated in the first dimension by IEF and in the second dimension by SDS-PAGE followed by staining with Coomassie Blue (A). The rectangle marks the area of specific reaction with PAB-positive sera in immunoblotting/ECL. The corresponding gel area with an apparent molecular weight of 80 kDa and an apparent isoelectric point of pH 4.8 was excised and analysed by MALDI-TOF mass spectrometry. Immunoblot analysis of polypeptides obtained from homogenised rat pancreas tissue separated by two-dimensional electrophoresis (B). Separated proteins were tested with PAB-positive human sera, and reactive molecules were visualised by ECL. The arrow marks the spot where a specific reaction occurred. Immunoblot analysis of pancreatic polypeptides tested with a PAB-negative serum of a patient with Crohn’s disease as negative control (C). ECL, enhanced chemiluminescence; IEF, isoelectric focusing; MALDI-TOF, matrix-assisted laser desorption ionisation time-of-flight; PAB, pancreatic autoantibody; SDS-PAGE, sodium dodecylsulfate polyacrylamide gel electrophoresis.
Identification of GP2 by MALDI-TOF mass spectrometry in all excised gel areas: On the peptide mass fingerprint, stars label the peaks for which the mass fits the mass of tryptic peptides from rat GP2 with a mass accuracy below 30 ppm. The insert shows the MS/MS spectrum with characteristic y ions confirming the sequence of one GP2 peptide. The high peaks of y4 and y6 further confirm the sequence because of the predictable weak peptide bonds before P and after E. The Mascot ion score was 106 and sequence coverage 25%. GP2, glycoprotein 2; MALDI-TOF, matrix-assisted laser desorption ionisation time-of-flight.
Reactivity of PABs to GP2 by ELISA
To further confirm the antigen specificity of PAB to GP2, zymogen granules were isolated from the pancreas of fasted Wistar rats, and GP2 was purified as described previously (fig 3).18 Sera from 42 PAB IgG-positive and 31 PAB IgG-negative patients with Crohn’s disease were analysed for IgG reactivity to solid-phase GP2 by ELISA and compared to sera from 49 patients with ulcerative colitis and 69 healthy blood donors (fig 4). PAB-positive sera from patients with Crohn’s disease displayed significantly higher IgG reactivity to GP2 (median OD, 1.025; 95% CI, 0.729 to 1.640) than either PAB-negative sera from patients with Crohn’s disease (median OD, 0.133; 95% CI, 0.094 to 0.176, p<0.0001), or sera from patients with ulcerative colitis (median OD, 0.174; 95% CI, 0.143 to 0.310, p<0.0001), or sera from healthy blood donors (median OD, 0.126; 95% CI, 0.089 to 0.179, p<0.0001; fig 4). In fact, 29 out of 42 (69%) patients with Crohn’s disease who were PAB-positive had elevated anti-GP2 IgG autoantibodies with an OD higher than 0.679 (95th percentile of blood donor OD). In contrast, only one patient with Crohn’s disease who was PAB-negative had an OD higher than 0.679. Remarkably, three out of five patients with ulcerative colitis and with elevated anti-GP2 IgG (OD>0.679) and one out of three healthy blood donors with elevated anti-GP2 IgG (OD>0.679) also tested positive for PAB by IIF.
Purification of rat GP2. SDS-PAGE of isolated zymogen pancreatic granules (B) prepared as described in Patients and Methods according to Ronzio et al18 and GP2 (C) after solubilisation by phosphoinositol-specific phospholipase C. The GP2 fraction (black arrow) showed purity greater than 90% according to the SDS-PAGE image. Molecular weight standards are run in lane (A). GP2, glycoprotein 2; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Median OD of anti-GP2 IgG antibodies determined by an ELISA employing purified rat GP2. The anti-GP2 IgG antibodies in serum samples from patients with Crohn’s disease who were PAB-positive differed significantly from those of patients with Crohn’s disease who were PAB-negative (p<0.0001), patients with ulcerative colitis (p<0.0001), and blood donors (p<0.0001). (A) Patients with Crohn’s disease who were PAB-positive (n = 42). (B) Patients with Crohn’s disease who were PAB-negative (n = 31) (C) Patients with ulcerative colitis (n = 49). (D) Blood donors (n = 69). ELISA, enzyme-linked immunosorbent assay; GP2, glycoprotein 2; IgG, immunoglobulin G; OD, optical density; PAB, pancreatic autoantibody.
Confirmation of specificity of PAB to GP2 by IIF
In a subsequent IIF assay, purified rat GP2 significantly reduced the binding of PAB-containing sera to exocrine pancreatic tissue (fig 5A,B). Depending on the staining pattern of exocrine pancreas, two types of PAB can be distinguished.21 PAB I are characterised by a drop-like staining in the lumen of pancreatic acini, whereas PAB II demonstrate a fine speckled staining in the cytoplasm of acinar cells. Purified rat GP2 completely inhibited the intracellular staining of PAB II in acinar cells. Bovine serum albumin at the same concentration did not interfere with the intercellular staining of PABs. However, purified rat GP2 concentrations of up to 5 mg/l did not block PAB II staining of pancreatic luminal structures. Polyclonal anti-GP2 antibodies generated in hyperimmunised rabbits stained pancreatic zymogen granules as reported previously22 and produced a staining pattern similar to that of PAB (fig 5C).
Immunofluorescence staining of PAB-positive sera and polyclonal anti-GP2 antibodies. Staining pattern on simian pancreas of serum from a patient with Crohn’s disease who was PAB-positive, showing the subtype I pattern characterised by extracellular drop-like staining in the acinar lumen of pancreatic tissue sections (white arrow) and the subtype II pattern demonstrating a speckled staining of the cytoplasm of pancreatic acinar cells (black arrow) (A). Pre-incubation of PAB-positive serum with purified rat GP2 inhibited specific labelling of PABs, resulting in inhibition of intracellular staining of pancreatic acinar cells (B), but did not significantly affect reactivity of PABs to luminal structures (black arrow). Polyclonal rabbit antibodies to GP2 produced a staining pattern on simian pancreas similar to that produced by PABs from patients with Crohn’s disease (C). GP2, glycoprotein 2; PAB, pancreatic autoantibody.
Reactivity of PAB to human recombinant GP2 by IIF
To determine the reactivity of PAB to human recombinant GP2, transfected mammalian HEK293 cells and non-transfected cells as controls were fixed onto microscopy slides. Patient samples were analysed for IgG and IgA reactivity to human recombinant GP2 by IIF (table 1). Synthesis of human recombinant GP2 in transfected HEK293 cells was shown by reactivity with polyclonal anti-GP2 antibodies (fig 6A).
IgG and IgA reactivity of PAB-positive sera with recombinant human GP2 expressed in transfected mammalian HEK293 cells. Transfected and non-transfected HEK293 cells were fixed onto microscope slides and tested with patient sera and rabbit anti-GP2 antibodies by IIF. Synthesis of GP2 in transfected HEK93 cells was shown by reaction with rabbit polyclonal anti-GP2 antibodies (A1). Non-transfected HEK293 as negative control demonstrated no reaction with polyclonal anti-GP2 (A2). IgG reactivity of a PAB-positive Crohn’s disease serum on transfected HEK293 cells (B1) and non-transfected HEK293 cells as control (B2); IgA reactivity of a PAB-positive Crohn’s disease serum on transfected HEK293 cells (C1) and non-transfected HEK293 cells as control (C2). PAB-negative Crohn’s disease serum on transfected HEK293 cells (D1) and non-transfected HEK293 cells (D2). GP2, glycoprotein 2; IgA and IgG, immunoglobulin A and G, respectively; IIF, indirect immunofluorescence; PAB, pancreatic autoantibody.
GP2 specific IgG and IgA in patients with PAB-positive and PAB-negative Crohn’s disease, ulcerative colitis, and blood donors detected by IIF using GP2 transfected HEK293 cells
In fact, 28 out of 42 (66%) patients who were PAB-positive and had Crohn’s disease had IgG autoantibodies reactive to human recombinant GP2 (fig 6B). All reactive sera showed elevated IgG to rat GP2 in ELISA. Eighteen out of the 42 (43%) PAB-positive sera demonstrated IgA reacting with human GP2 synthesised by the transfected HEK293 cells (fig 6C). There was no specific reaction with non-transfected HEK293 cells. All 31 PAB-negative sera of patients with Crohn’s disease, including the patient with elevated anti-GP2 in ELISA, did not specifically react with human recombinant GP2 (fig 6D). Interestingly, serum samples from only one patient with ulcerative colitis (n = 49) and only one blood donor (n = 69), respectively, demonstrated IgG reactivity to recombinant human GP2 in IIF. Both sera demonstrated a positive PAB in IIF. There was no positive IgA reactivity to GP2 in either group.
GP2 expression in human colon biopsies
Semi-quantitative real-time PCR was used to assess GP2 mRNA synthesis normalised to the housekeeping gene β-actin in colon biopsies of Crohn’s disease (n = 4), ulcerative colitis (n = 4), and control patients (n = 5) (fig 7). GP2 fragments amplified with two different primer pairs from selected samples were confirmed by forward and reverse sequencing of PCR products. The GP2 transcript was found in all biopsies investigated except for two samples from patients with ulcerative colitis, whose GP2 Ct values were too low to reach the detection threshold. In detail, patients with Crohn’s disease demonstrated a significantly higher GP2 mRNA transcription level in colon biopsies (median Ct = 2.230; 95% CI, 0.354 to 4.089) than patients with ulcerative colitis in colon biopsies (median Ct = 0.049; 95% CI, −0.203 to 0.478; p = 0.0286). Control patients showed also a lower transcription level than Crohn’s disease patients (median Ct = 1.191; 95% CI, 0.715 to 1.667); however, the difference was not significant (p = 0.1905). Interestingly, the transcription level of GP2 in biopsies from patients with ulcerative colitis was also significantly lower compared to control biopsies (p = 0.0159). In fact, results from statistical analysis did not vary when GP2 values were normalised to the housekeeping gene GAPDH (data not shown).
GP2 mRNA transcription level assessment in human colon biopsies. Patients with Crohn’s disease (A) demonstrated a significantly higher GP2 mRNA transcription in relation to the housekeeping gene β-actin in colon biopsies assessed semi-quantitatively by rt-PCR than patients with ulcerative colitis (B, p = 0.0286). Control patients (C) showed a significantly higher transcription level in comparison with patients with ulcerative colitis also (B, p = 0.0159). Notably, statistical results after comparison of GP2 transcription levels did not vary when GP2 values were normalised to the housekeeping gene GAPDH (data not shown). (A) Patients with Crohn’s disease (n = 4). (B) Patients with ulcerative colitis (n = 4). (C) Control patients (n = 5). GADPH, glyceraldehyde-3-phosphate deyhydrogenase; GP2, glycoprotein 2; PCR, polymerase chain reaction.
Detection of GP2 in colon biopsies by IIF
Colon biopsies from patients with Crohn’s disease (n = 4), patients with ulcerative colitis (n = 4), and control patients (n = 5) were probed with human PABs by IIF to detect GP2. Three of four biopsies from patients with Crohn’s disease showed a positive staining of colonic enterocytes by strongly positive human PABs with a titre of 1 in 320 and higher (fig 8A). Biopsies from patients with ulcerative colitis and from control patients did not demonstrate a specific binding of PAB (fig 8B). PAB-negative sera did not react with the biopsy sections (fig 8C).
Immunofluorescence staining by pancreatic autoantibodies (PABs) of colon biopsy tissue sections from Crohn’s disease, ulcerative colitis, and control patients. PABs stained enterocytes of Crohn’s disease colon biopsy sections (A), whereas ulcerative colitis (B) and control colon biopsies (C) did not show a specific reaction with PAB.
Discussion
This study demonstrates for the first time that GP2 is the major autoantigen recognised by Crohn’s disease-specific PABs as shown by the interaction of PABs with purified rat GP2 in ELISA and recombinant human GP2 transiently expressed in mammalian HEK293 cells in IIF. Differences in the prevalence of human recombinant and native rat GP2 specific autoantibodies in PAB-positive sera could be due to differing amino acid sequences or post-translational changes like glycosylation. Glycoprotein 2 is a heavily glycosylated 78 kDa protein with N-linked carbohydrates. When conservative substitutions are considered, the human amino acid sequence of GP2 is 80% similar to that of the rat.23
Glycoprotein 2 reactive autoantibodies seem to recognise mainly conformational epitopes as reported for other pancreas specific autoantibodies found in patients with type 1 diabetes.24 Only patients with high concentrations of PABs showed reactivity to GP2 in immunoblotting experiments.
Glycoprotein 2 accounts for up to 40% of all zymogen granule membrane proteins in pancreatic acinar cells and is linked to the granule membrane via a glycosyl phosphoinositol (GPI) anchor.18 25 26 Upon hormonal or neuronal stimulation of the pancreas, GP2 is transported to the apical compartment of acinar cells, from which it is released together with zymogens into the pancreatic duct.22 27 In IIF assays, PAB stain different structures of the exocrine pancreas and are divided into two subtypes accordingly.21 Subtype I PABs exhibit the typical extracellular drop-like staining pattern in the acinar lumen of pancreatic tissue sections and PABs of subtype II demonstrate a speckled staining of the cytoplasm of pancreatic acinar cells.19 This is consistent with the localisation of GP2 in the intercellular zymogen granules and in the pancreatic ducts after its release together with zymogens, respectively.27
As a self-binding glycoprotein, GP2 forms soluble aggregates in pancreatic juice after cleavage;22 this is consistent with the presumed high molecular weight of the sought pancreatic juice protein.9 Furthermore, pancreatic juice was found to inhibit the binding of PAB on human pancreatic tissue in IIF assays.9
The physiological function of GP2 remains enigmatic. Glycoprotein 2 is presumed to influence granule formation by interacting with syncollin, zymogen granules ZG16p and ZG46p, and proteoglycans in a submembranous matrix.28 However, a GP2-deficient mouse model recently showed that GP2 is not essential for exocrine secretion and zymogen granule formation.29
The question is therefore: Do anti-GP2 autoantibodies or GP2 itself play a pathophysiological role in Crohn’s disease, or are anti-GP2 autoantibodies merely an epiphenomenon since they do not recognise intestinal antigens? GP2 is reportedly associated with the cholesterol–glycosphingolipid-enriched microdomains (lipid rafts) of secretory granule membranes of the pancreatic acinar cells of rats.30 Contrary to data describing GP2 as a pancreas-specific protein, GP2 has also been found in lipid rafts of the brush-border membrane of small intestinal enterocytes of rats.31 Furthermore, GP2 has been shown to be specifically synthesised in murine M cells of Peyer’s patches.15 M cells and Peyer’s patches, which are particular abundant in the distal part of the ileum, are suggested to be potential sites of the inflammatory onset in Crohn’s disease.32 33
In this study we were able to confirm GP2 expression at mRNA and protein levels in colon biopsies from patients with Crohn’s disease for the first time. Therefore, autoimmunity to GP2 can theoretically result in targeting of the intestine, the organ mainly affected by Crohn’s disease.
Interestingly, chronic pancreatitis that occurs as a rare extra-intestinal complication of Crohn’s disease differs from that observed in ulcerative colitis.34 The use of elevated serum GP2 levels as serological markers for acute and chronic pancreatitis has been proposed.35 In Crohn’s disease, however, PABs occur far more frequently than clinically symptomatic chronic pancreatitis, and the frequency of chronic pancreatitis is virtually the same in patients with Crohn’s disease who are PAB-positive and PAB-negative.9 Furthermore, GP2 knock-out mice with two types of experimentally induced pancreatitis did not differ significantly from wild-type mice.29
In addition to IgG and IgM PAB isotypes, IgA pancreatic autoantibodies have also been detected in patients with Crohn’s disease.9 In this study, we were also able to detect IgA autoantibodies to GP2 in PAB-positive Crohn’s disease sera. IgA pancreatic autoantibodies may interact with enterocytic cell GPI-anchored GP2 or with GP2 in pancreatic juice secreted into the intestinal lumen. Whether this may contribute to the induction or perpetuation of mucosal inflammation remains to be shown.
Interestingly, the rat GP2 amino acid sequence exhibits a 53% identity and 85% similarity to human Tamm–Horsfall protein (THP; uromodulin) over a 450 amino acid stretch covering all 28 cysteins. THP, a likewise GPI-anchored 85 kDa glycoprotein, is synthesised in cells of the ascending limb of the loop of Henle.36 Autoantibodies to THP have been observed in urinary tract inflammation, but their disease specificity is disputed.37 Genes of the two glycoproteins constitute a family of homologous genes thought to be generated by duplication of a common ancestor gene that evolved separately to acquire tissue specificity.38 Although it is the most abundant urine protein, the function of THP remains unclear. However, defective THP synthesis has been shown to increase the susceptibility of mice to urinary tract infections.39 THP binds to uropathogenic type1 fimbriated Escherichia coli and keeps bacteria from interacting with urothelial (uroplakin) receptors.40
Although experimental evidence that soluble or membrane-anchored GP2 reacts with bacteria is lacking, GP2 secreted by the pancreas and not digested by zymogens could theoretically block the interaction of adhesive bacteria in the intestinal tract like THP does in the urinary tract. No specific pathogenic species has been associated with Crohn’s disease, but high concentrations of mucosal microbes, especially adhesive bacteria, have been detected in Crohn’s disease patients.41 There is an increased risk for development of IBD after gastrointestinal infections.42
THP has also been thought to be a regulatory factor of innate and adaptive immunity of the urinary tract by interacting with toll-like receptor 4 (TLR-4).43 TLR-4 is strongly upregulated in enterocytes of patients with Crohn’s disease.3 TLR4 Asp299Gly polymorphism is associated with Crohn’s disease and TLR-4 mutations influence antibody formation against microbial epitopes in patients with Crohn’s disease.4 44 As an important result, our data indicate that GP2 expression is induced in the targeted tissue of patients with Crohn’s disease. However, due to the limited number of patients and controls, further studies are required to clarify whether GP2 expression is a disease-specific process in the intestine of patients with Crohn’s disease.
In summary, the present study provides evidence that GP2 is a major and specific autoantigen of PABs in Crohn’s disease. Furthermore, GP2 was detected in human colon biopsies at the site of inflammation in Crohn’s disease. Further investigation of the role of GP2 in the aetiopathogenesis of Crohn’s disease is warranted. This novel autoantigen may trigger progress in understanding the aetiology of intestinal disorders and facilitate resolving the remaining mysteries of IBD.45
Acknowledgments
We thank M Schmid for excellent technical assistance in mass spectrometry.
REFERENCES
Footnotes
Funding This work was supported by the Brandenburg Ministry of Economics and European Union grant 80130073.
Competing interests DR is a shareholder of GA Generic Assays GmbH. TP is a shareholder of both Seramun Diagnostica GmbH and GA Generic Assays GmbH. Both companies are diagnostic manufacturers. The remaining authors have no competing interests.
Provenance and peer review Not commissioned; externally peer reviewed.
Ethics approval This study was approved by the local ethics committee of the Medical Faculty, Technical University Dresden, on 3 May 2007. The animals used in this study were handled according to German regulations concerning the protection of animals.
Linked Articles
- Digest