Article Text
Abstract
Background—Standard treatment of inoperable hepatocellular carcinoma has not been established. Somatostatin has been shown to possess antimitotic activity against a variety of non-endocrine tumours.
Aims—To assess the presence of somatostatin receptors in human liver and to treat advanced hepatocellular carcinoma with the somatostatin analogue, octreotide.
Methods—Somatostatin receptors were measured in liver tissue homogenates from patients with acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Fifty eight patients with advanced hepatocellular carcinoma were randomised to receive either subcutaneous octreotide 250 μg twice daily, or no treatment. Groups were comparable with respect to age, sex, Okuda classification, presence of cirrhosis, and liver biochemistry and virology.
Results—Various amounts of somatostatin receptors were identified in liver tissue of all patients including those with hepatocellular carcinoma. Treated patients had an increased median survival (13 months versus four months, p=0.002, log rank test) and an increased cumulative survival rate at six and 12 months (75% versus 37%, and 56% versus 13% respectively). Octreotide administration significantly reduced α fetoprotein levels at six months. When a multivariable Cox’s proportional hazards model was fitted, variables associated with increased survival were: treatment administration, absence of cirrhosis, increased serum albumin, and small tumours. Treated patients clearly had a lower hazard (0.383) in the multivariate analysis.
Conclusions—Octreotide administration significantly improves survival and is a valuable alternative in the treatment of inoperable hepatocellular carcinoma.
- hepatocellular carcinoma
- octreotide
- somatostatin receptors
- liver disease
Statistics from Altmetric.com
Hepatocellular carcinoma (HCC) is widely distributed in different geographical areas. There is a high prevalence in Asia and Africa1 whereas the prevalence is very low in Western societies.2 In Greece, the annual death rate has been estimated to be 10–23 per 100 000 population per year.3
Treatment of inoperable HCC remains unsatisfactory and different therapeutic regimes have been tested. Recently trials with tamoxifen,4 flutamide,5 and chemoembolisation with lipiodol6-9 have been reported.
Somatostatin, a hormone with well proven efficacy for the treatment of hormone producing tumours10 has been tested for efficacy in non-hormone producing tumours and somatostatin receptors have been identified in various adenocarcinomas including breast, kidney, large bowel, and ovary.11 ,12 Somatostatin receptors in human liver have not been adequately studied.
The present study had two aims: to study somatostatin receptors in human liver tissue in hepatic diseases; and to assess the clinical efficacy of octreotide administration in the treatment of HCC.
Patients and methods
SOMATOSTATIN RECEPTORS
Needle liver biopsy specimens from 23 patients were used for somatostatin receptor determination. Histological diagnoses were: acute hepatitis (four patients), chronic hepatitis (nine patients), cirrhosis (six patients), and HCC (four patients).
Liver biopsy specimens were homogenised in Tris-HCl buffer, pH 7.4, containing 200 μg/ml bacitracin, 10 μg/ml leupeptin, 5 mM MgCl2, 5 mM EGTA and 0.1 M phenylmethylsulphonyl fluoride (buffer I) using a Polytron (Ultra-Turrax T-25) homogeniser. Due to the small quantity of tissue, the homogenate was used immediately for binding studies without any further membrane preparation. The amount of protein in each sample was determined according to the Bradford method.13
The concentration of somatostatin receptors in the biopsy specimens sampled was determined as previously described.14 ,15Biopsy specimen homogenates were incubated for 90 minutes at 25°C with iodine-125 labelled tyr11-somatostatin (Amersham, specific activity 2000 Ci/mmol; 1.5 nM) and buffer I in a final volume of 500 μl. The binding reaction was terminated by vacuum filtration over GF/C filters (Whatman) presoaked with 0.1% polyethylenamine for 30 minutes and 1% bovine serum albumin for 60 minutes. Filters were washed with 15 ml cold Tris-HCl buffer, pH 7.4, and the bound radioactivity was measured in a gamma counter (LKB 75% efficiency). Different concentrations (100 nM, 1, 10, and 20 μM) of somatostatin-14 or somatostatin-28 were used to define specific binding for somatostatin receptors. No difference in the specific binding was observed between the three concentrations of the peptide and the prohormone.
THERAPEUTIC STUDY
Fifty eight patients were included in the therapeutic trial between 1992 and 1996. Inclusion criteria were liver biopsy diagnosis of HCC and/or increased levels of α fetoprotein (AFP) over 500 ng/l with compatible liver ultrasound, computed tomography scan, or hepatic angiography. Patients with small tumours judged to be suitable for surgery were excluded. Patients with variceal bleeding or hepatic encephalopathy during the last 30 days were also excluded. All patients were classified according to Okuda staging,16 ,17 while cirrhotics were also classified according to Child-Pugh criteria. Patient consent for participation in the study was obtained.
TREATMENT SCHEDULE AND FOLLOW UP
All patients included in the study were randomised into two groups with the random number method. The first group received 500 μg of octreotide (Sandostatin, Sandoz Hellas) subcutaneously in two divided doses. Treatment was given until patient withdrawal or death. The second group received no treatment and served as controls.
All patients had a monthly follow up with routine liver biochemical tests; every two months AFP concentrations were determined and a liver ultrasound was performed every three months. All biochemical tests were performed at the central hospital laboratory. Hepatitis B virus markers were detected with commercial enzyme linked immunosorbent assay (ELISA) kits (Abbott) while an ELISA II or III was used for anti-HCV detection. A radioimmunoassay method was used for AFP estimation. All percutaneous liver biopsies were ultrasound guided.
STATISTICAL ANALYSIS
Statistical analysis was performed by standard methods.18-20 Ten possible prognostic variables were recorded: age; sex; place of residence; serum albumin; serum bilirubin; presence of cirrhosis; presence of hepatitis B surface antigen (HBsAg), anti-HCV (hepatitis C virus) or both; size of tumour (small, medium, large, multiple); AFP (initial and repeated AFP measurement); and treatment or no treatment. The two classifications (Child-Pugh A, B, C and Okuda I, II, III) were also used. Survival times were recorded in months. Initially, the χ2 approach was used to test the homogeneity of the treatment and control groups with respect to the prognostic factors.
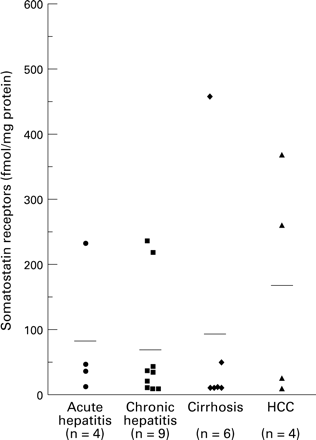
Somatostatin receptor levels in the four histological groups are displayed using dot plots and were tested using a non-parametric approach.
As the distribution of AFP concentration values was highly positively skewed, logarithms were taken of three values before any analyses were performed. The resulting variables had an approximately normal distribution.
Two approaches were used in the survival analyses. Firstly, Kaplan-Meier survival curves21 were plotted for each factor and the corresponding log rank tests were performed, on the null hypothesis that the risk of death is the same for each level of the factor. The continuous variables—age, AFP, bilirubin, and albumin—were each grouped into a three level factor, with approximately equal numbers in each level, for appropriate Kaplan-Meier curves to be plotted and log rank tests performed. Secondly, a multivariable Cox proportional hazards model22 was fitted using stepwise regression procedures (likelihood ratio tests) to determine significant variables.
Results
SOMATOSTATIN RECEPTORS
In the 23 patients classified according to the histological groupings of acute hepatitis, chronic hepatitis, cirrhosis, or HCC, receptor levels were either low (less than 50 fmol/mg) or high (greater than 210 fmol/mg) (fig 1) Median values in the four groups were 34.0 (range 5.5–226), 28.0 (3–320), 5.25 (4.5–453), and 137 (4.5–363) fmol/mg protein. There is no evidence of a difference in the mean levels between groups (Kruskal-Wallis test, p=0.656, χ2=1.61 on 3 df) but the power of the test is low as the sample sizes are small.
Somatostatin receptors in liver tissue. Patients with either low or high levels of receptors can be identified in all disease groups. HCC, hepatocellular carcinoma.
THERAPEUTIC TRIAL
The data consist of observations on 58 HCC patients. There were 56 deaths and two censored observations. Twenty eight patients were randomised to the treatment group and 30 patients served as the control group. Analysis was by intention to treat. There were four patient withdrawals from the treatment group (none from the control group). The mean (SEM) length of follow up was 14.1 (2.17) months (range 1–42 months) for the treated patients and 6.6 (1.36) months (range 1–33 months) for the controls. There were no major dissimilarities in the treatment group regarding the prognostic variables (χ2test) (table 1).
Clinical and laboratory data of patients groups. No differences were found (χ2 test)
Kaplan-Meier survival curves
At the time of the final analysis (May 1996) only two patients (both from the treatment group) were alive. Table 2 shows the percentage of patients surviving six and 12 months. Log rank test results for the following factors were significant:
Cumulative six and 12 month survival rates for the prognostic variables
Treatment (p=0.0024, df=1). There is a significant survival difference between those who underwent treatment and those who did not. The median survival time for those patients who received treatment was 13.0 (1.90) months (95% confidence interval 9.3 to 16.7 months) while for those who did not receive treatment it was 4.0 (1.10) months (95% confidence interval 1.9 to 6.2 months). Thirty seven per cent of non-treated and 75% of those treated were alive at six months. At 12 months the figures were 13% and 56% and at 24 months 3% and 20% respectively (fig 2).
Survival curves for treated and non-treated patients with HCC.
Cirrhosis (p=0.0285, df=1). This is significant evidence that those patients with cirrhosis have a higher instantaneous risk of death than those without cirrhosis.
Survival probability for treated versus non-treated cirrhotics with HCC is shown in fig 3 (p=0.0114, df=1).
Survival curves for treated and non-treated cirrhotics with HCC.
Albumin (p=0.0161, df=2). Higher albumin concentrations are associated with a decreased hazard rate.
Okuda classification (p=0.020, df=2). Okuda I patients have a median survival time of 16.0 months (SE 9.00) whereas Okuda II and Okuda III patients have median survival time of 7.0 months (SE 2.24 and 0.78 respectively).
To test the effectiveness of the drug in patients while controlling for good and poor prognostic factors, log rank tests were performed controlling for Okuda I/II versus III and small/medium versus large/multiple tumours, for treated and untreated patients. Table 3presents subgroup results. Treatment is effective after adjustment for Okuda staging or tumour size.
Comparison of survival distributions of treated patients versus controls while controlling for Okuda grouping (log rank p = 0.0134) and tumour size (long rank p = 0.0085)
Multivariate analysis
The final model fitted contains the following variables: treatment, log (AFP), albumin, cirrhosis, and tumour size (table 4). Treated patients have a lower hazard (0.383) than those who did not undergo treatment, accounting for the prognostic variables. A unit increase in the albumin concentration decreases the hazard rates by 10.1%, all other covariates remaining unchanged (as the ratio of hazard rates for cases one unit apart is 0.899). The relative risk for those with cirrhosis is 5.47—that is, estimated risk of dying is 5.47 times greater for those with cirrhosis, adjusting for the other covariates. As the concentrations of albumin and AFP increase, the hazard decreases. Having medium, large, and multiple tumours is associated with a higher risk of death compared with those with small tumours.
Multivariate analysis for significant variables
Effect of treatment on tumour size
Small satellite tumours (below 3 cm in diameter) disappeared after six to 12 months of treatment in five patients (fig 4). In four additional patients, the size of the tumour remained unchanged during treatment. In all other treated patients and in all untreated patients the size gradually increased.
Liver ultrasound of a patient before (top panel) and after six months (bottom panel) of treatment with octreotide. Small satellite tumours have regressed while the large tumour remains unchanged.
Influence of treatment on α fetoprotein concentration
Figure 5 shows the effect of treatment for those surviving for 6, 12, 18, or 24 months of treatment. Thirteen out of 20 had a reduction in AFP after treatment, in some instances greater than 50% of the initial value. When pairwise comparisons were made of the initial AFP concentrations with those at six months (using the non-parametric Wilcoxon signed ranks test), there was evidence of a systematic difference between the measurements (p=0.039). There were too few 12 month readings for a repeated measures analysis.
AFP concentration in the treated group. M, months.
A log rank test was performed to test for differences in the survival distributions of those patients who had undergone treatment and had survived for at least six months, according to whether their AFP concentration had increased or decreased. There was no evidence of a difference (p=0.365) for increased AFP (median 13 months, SE 1.85), or for decreased AFP (median 14 months, SE 0.65). In the control group AFP remained either constant or gradually increased.
Adverse effects
The drug was well tolerated. A mild diarrhoea was noted at the beginning of the treatment in 40% of the patients but this subsided on continuation of treatment. The only difficulty was the need for twice daily subcutaneous injections, which was the reason for discontinuation of treatment of four patients after four to six months.
Quality of life
Appetite, body weight, and the general feeling of well being were used as criteria: 15 of the 28 (53.6%) patients reported a remarkable improvement in the feeling of well being, while an increase in appetite was reported in 24 (85.7%) treated patients. Body weight improved in 12 (42.8%) of the patients treated. No differences were noted in the control group.
Discussion
Response of HCC to different therapeutic modalities is poor and no firm general recommendations can be made.23 A new therapeutic approach for advanced HCC has been investigated in the present study. Somatostatin is a hormone with known antimitotic activity in various neoplasms. Synthetic analogues have been shown to delay tumour growth in animals.24 The presence of somatostatin receptors in liver tissue, however, has not been adequately studied. A study of somatostatin receptor subtypes in rats reported the presence of type 3 receptors in the liver without data on other subtypes.25 In a study using monoclonal antibodies in rat liver,26 detection of subtype 2 receptor was not reported.
OctreoScan indium scintigraphy has also produced conflicting results. One study reports very low levels of liver uptake in rats.27 Similar studies in humans reported either uptake by the liver without further details28 or very low uptake.29
In our study we have investigated the presence of somatostatin receptors in human diseased livers using a technique that detects all receptor subtypes. We found two distinct groupings of somatostatin receptors, unrelated to the underlying liver pathology. These findings could be attributed to fluctuation of the total receptor levels, to different subtype preponderance among individuals, or to a heterogeneous distribution of somatostatin receptors. The latter finding has been reported in certain adenocarcinomas,11pituitary adenomas,30 and carcinoid tumours.31 Further studies are required to clarify the issue. The presence of somatostatin receptors led to the clinical trial of octreotide administration for the treatment of advanced HCC.
Results have clearly shown a significant improvement in both median survival time (13 versus four months) compared with the control group and estimated survival rates after 6, 12, 18, 24, and 30 months of follow up. Tolerance to treatment was very good. In particular no problems with renal function were noted, a complication that has been reported in cirrhotic patients.32
Improvement in quality of life was of particular interest in the present study. Although quality of life is very difficult or even impossible to assess objectively, the criteria used were improvement of appetite and increase in body weight (not related to ascites). A total of 53.6% of patients in the treated group and none in the controls had a positive response. Moreover, the general sense of well being improved dramatically in the treated group although the validity of this observation was limited by the fact that the study was not double blind.
In five treated patients small satellite tumours (diameter less than 3 cm) disappeared, while in four more patients, larger tumours (3–6 cm diameter) remained unchanged. No similar observations were found in the control group. AFP levels were reduced after six months of treatment.
Other therapeutic modalities for advanced HCC include lipiodol embolisation5 ,7 ,8 or hormonal manipulations.4 ,5 In a recent report6intra-arterial iodine-131 labelled lipiodol administration gave comparable results to our study regarding tumour regression, but median survival and estimated survival rates at six and 12 months were higher in our study.
Our study also has more favourable results than those of a study on mainly Okuda II tumours treated with either lipiodol-epirubicin or131I-lipiodol.32 Only when the majority of patients are Okuda I are the estimated survival rates better than ours although the median survival time is similar to ours.33
In a recent study, Greek patients have been treated with tamoxifen.4 Median survival time and estimated survival rates are better in the present study group compared with tamoxifen treated patients (22% survival after 12 months compared with 58% in the present study). The Cox analysis showed no sex effect in our study as opposed to the tamoxifen study. However, this may be attributed to the fact that there were few women in our study. It should be noted that in both the lipiodol group and the tamoxifen group, the percentage of Okuda III tumours was lower than in the present study. In a separate study, the antiandrogen drug flutamide given to a group of advanced HCC patients with similar characteristics to our treated group showed no therapeutic effect, with a median survival time of 2.5 months.5
More favourable results than those in our study have only been reported in a recent paper of 122 cirrhotic HCC patients with resected tumours34; an 80% 12 month survival rate is reported. However, the majority were Okuda I tumours, while survival of those with a tumour larger than 5 cm is only 51% at 12 months (which is lower than in our patients).
The mechanism of octreotide antimitotic activity has not been explored in the present study. It could be either direct through receptor subtypes 2 and 58 ,11 ,12 ,35-38 or indirect through suppression of tumour trophic hormones such as insulin or insulin-like growth factor 1 (IGF-1).39
In rats, octreotide administration caused a reduction in regeneration after partial hepatectomy40 ,41 due to suppression of insulin secretion. This latter effect is improbable in HCC patients because in human HCC cell cultures a fourfold increase in expression of IGF-1 binding protein has been reported and therefore an increased effect of IGF-1 on HCC is to be expected.42 Evidence against the indirect effect is also the reported regression of hepatic adenocarcinoma metastases in rats without detectable effects on serum growth hormone, prolactin, and IGF-1. Of greatest importance is the fact that in this study only metastases bearing somatostatin receptors were arrested.43
It has been reported recently that human HCCs express receptors for cholecystokinin B and gastrin, two hormones that could be trophic for the tumour.44 Despite previous reports suggesting that there is no indirect effect it is still possible that somatostatin antimitotic activity in HCC might be partly related to suppression of these two hormones.
In conclusion, octreotide administration proved beneficial for the survival of patients with advanced HCC without serious adverse effects. Octreotide treatment is therefore a valuable alternative therapy for inoperable HCC. The expected availability of long acting somatostatin analogues might be very useful for this condition.
References
Linked Articles
- COMMENTARY