Article Text
Abstract
Background—Both acid and duodenal contents are thought to be responsible for the mucosal damage in Barrett’s oesophagus, a condition often treated medically. However, little is known about the effect of omeprazole on duodenogastric reflux (DGR) and duodenogastro-oesophageal reflux (DGOR).
Aims—To study the effect of omeprazole 20 mg twice daily on DGR and DGOR, using the technique of ambulatory bilirubin monitoring.
Methods—Twenty three patients with Barrett’s oesophagus underwent manometry followed by 24 hour oesophageal and gastric pH monitoring. In conjunction with pH monitoring, 11 patients (group 1) underwent oesophageal bilirubin monitoring and 12 patients (group 2) underwent gastric bilirubin monitoring, both before and during treatment with omeprazole 20 mg twice daily.
Results—In both groups there was a significant reduction in oesophageal acid (pH<4) reflux (p<0.005) and a significant increase in the time gastric pH was above 4 (p<0.005). In group 1, median total oesophageal bilirubin exposure was significantly reduced from 28.9% to 2.4% (p<0.005). In group 2, median total gastric bilirubin exposure was significantly reduced from 24.9% to 7.2% (p<0.005).
Conclusions—Treatment of Barrett’s oesophagus with omeprazole 20 mg twice daily results in a notable reduction in the exposure of the oesophagus to both acid and duodenal contents. In addition, delivery of duodenal contents to the upper gastric body is reduced.
- bilirubin monitoring
- Barrett’s oesophagus
- omeprazole
- pH monitoring
- duodenogastric reflux
- duodenogastro-oesophageal reflux
Statistics from Altmetric.com
- bilirubin monitoring
- Barrett’s oesophagus
- omeprazole
- pH monitoring
- duodenogastric reflux
- duodenogastro-oesophageal reflux
Barrett’s oesophagus is a premalignant condition associated with severe, chronic oesophageal reflux of gastroduodenal contents.1 Although there is doubt as to the relative contributions of acid and duodenal contents to the pathogenesis of Barrett’s oesophagus, experimental studies have shown them to cause intestinal metaplasia alone2-4 and in combination.3 ,5 Ideally, therefore, treatment should be aimed at eliminating both acid and bile gastro-oesophageal reflux. By reconstructing the defective lower oesophageal sphincter, antireflux surgery has been shown to achieve this.6 A large proportion of patients with Barrett’s oesophagus, however, are symptomatically well maintained on proton pump inhibitors (PPI). While the profound effect of PPIs on gastro-oesophageal acid reflux has been well documented, the effect of PPIs on duodenogastro-oesophageal reflux (DGOR) has only been reported in one study, which showed a reduction of DGOR by omeprazole in six of nine patients.7 Indeed, by producing an alkaline environment in the oesophagus and stomach, it has been suggested that omeprazole may enhance the damaging effects of bile salts.8
The technique of ambulatory bilirubin monitoring in combination with pH monitoring allows the continuous simultaneous assessment of duodenogastric reflux (DGR) or DGOR and pH. We hypothesised that treatment with omeprazole 20 mg twice daily would effectively eliminate acid reflux, but would leave DGR and DGOR unchanged in an alkaline environment. Patients in this unit with Barrett’s oesophagus are routinely asked to undergo repeat 24 hour pH monitoring while taking acid suppression medication to confirm elimination of acid reflux. The aim of this study was to determine the effect of omeprazole 20 mg twice daily on DGR and DGOR in patients with Barrett’s oesophagus.
Patients and methods
PATIENTS
Twenty three patients with Barrett’s oesophagus were recruited from routine referrals to the Oesophageal Laboratory. Barrett’s oesophagus was defined as replacement of 3 cm or more of the tubular oesophagus by columnar epithelium with histologically proved intestinal metaplasia. No patient had had previous upper gastrointestinal surgery. A detailed history was taken from each patient.
The patients were divided into two groups. All patients underwent manometry followed by 24 hour dual oesophageal and gastric pH monitoring. In conjunction with dual pH monitoring, 11 patients (group 1) underwent oesophageal bilirubin monitoring and 12 patients (group 2) underwent gastric bilirubin monitoring. All patients underwent 24 hour monitoring twice: firstly while not taking omeprazole (treatment was stopped at least seven days beforehand); and secondly after 6–10 weeks of treatment with omeprazole 20 mg twice daily, while on treatment. No patient was receiving prokinetic medication.
Ideally, all patients would have undergone ambulatory pH and bilirubin monitoring of both oesophagus and stomach. However, it was considered unacceptable to subject patients either to four separate 24 hour studies, or to simultaneous pH and bilirubin monitoring of both oesophagus and stomach (which would have involved three transnasal catheters). Therefore the effect of omeprazole on DGR and DGOR was investigated in two different groups of patients with Barrett’s oesophagus.
MANOMETRY AND 24 HOUR pH AND BILIRUBIN MONITORING
Static manometry was performed transnasally using a solid state pressure catheter (Gaeltec Ltd, Isle of Skye) in order to determine the position of the lower oesophageal sphincter. pH monitoring was performed using a dual channel antimony pH catheter (Synectics Medical Ltd, Sweden) with the sensors 15 cm apart. Bilirubin monitoring was performed using a fibreoptic bilirubin sensor (Bilitec 2000, Synectics Medical Ltd, Sweden). Bilitec 2000 relies on the fact that bilirubin has a characteristic absorption peak at 450 nm, and in this way uses bilirubin as a marker of the harmful components of bile (bile salts and trypsin). The system has been extensively validated both in vitro and in vivo.9-12 Prior to use, the pH sensors were calibrated in buffers of pH 7 and pH 1 and the bilirubin sensor in water. The pH and bilirubin catheters were passed transnasally. Oesophageal pH and bilirubin sensors were placed 5 cm above the upper border of the lower oesophageal sphincter. Gastric pH and bilirubin sensors were placed 15 cm distal to the proximal sensor, in the upper gastric body. Subjects were given a previously validated thorough list of dark coloured foodstuffs to avoid (for example, tea, coffee, coca cola, red meat, spinach, carrots, tomato soup) which absorb light at a similar wavelength to bilirubin. After 24 hours, the sensors were removed, calibrated, and the data downloaded and analysed using commercially available software (EsopHogram, Synectics Ltd, Sweden).
Acid reflux was defined as occurring when the oesophageal pH fell below 4.13 Bilirubin was defined as being present when the absorbance was greater than 0.14.14 The percentage time gastric pH was greater than 4 was calculated. Although this value is arbitrary, it has been the most commonly used pH value for estimation of DGR.15-17 Pathological acid reflux was defined as a total oesophageal acid exposure time greater than 5.8% and pathological oesophageal and gastric bilirubin reflux were defined as total bilirubin exposure time greater than 7% and 35% respectively (values based on the 95th percentile of normal subjects studied in our laboratory).
STATISTICAL ANALYSIS
All values are expressed as median (range). Comparisons between groups were made using the Mann-Whitney U test and Fisher’s exact test where appropriate. Comparisons within groups were made using the Wilcoxon sign rank test. A p value less than 0.05 was considered significant.
Results
PATIENT DEMOGRAPHICS
Table 1 shows the demographics of group 1 (oesophageal bilirubin monitoring) and group 2 (gastric bilirubin monitoring). There was no significant difference between the groups with respect to age (p=0.8), sex distribution (p=0.45), length of Barrett’s mucosa (p=0.73), or lower oesophageal sphincter pressure (p=0.06). In addition, there was no significant difference in total oesophageal acid exposure time between the groups when off omeprazole (p=0.62).
Patient characteristics in group 1 (oesophageal bilirubin monitoring) and group 2 (gastric bilirubin monitoring)
REFLUX TIMES
Table 2 shows the median percentage total acid and bilirubin reflux times and median percentage total time gastric pH was above 4 for both groups, both off and on omeprazole 20 mg twice daily. In both groups, median total oesophageal acid reflux times were significantly reduced by omeprazole (p<0.005). Median total oesophageal bilirubin exposure time (group 1) was significantly reduced by omeprazole (p<0.005). Median total gastric bilirubin exposure time (group 2) was also significantly reduced by omeprazole (p<0.005). The percentage time gastric pH was above 4 was significantly increased by omeprazole (group 1, p<0.05; group 2, p<0.005).
Percentage total oesophageal acid (pH<4) and bilirubin (absorption >0.14) reflux times, gastric bilirubin (absorption > 0.14) reflux times, and percentage time gastric pH>4, both off and on omeprazole treatment
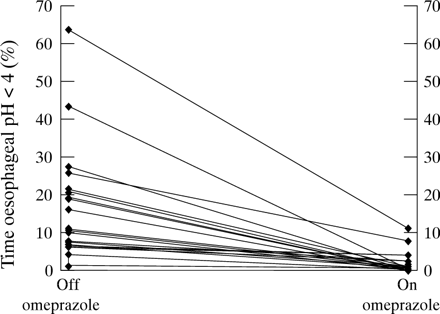
Figures 1, 2, and 3 show the effect of omeprazole 20 mg twice daily on individual acid, oesophageal bilirubin, and gastric bilirubin reflux times respectively for each patient. There was a reduction in oesophageal acid exposure in all patients (fig 1). Only two patients had pathological acid reflux on treatment. In all but one patient, oesophageal bilirubin exposure (group 1) was reduced by omeprazole (fig3). In one patient, oesophageal bilirubin exposure increased slightly within normal limits (from 5.1% to 7.1%). In one patient, omeprazole notably reduced oesophageal bilirubin exposure but failed to return it to within normal limits (61% to 14.9%). Omeprazole resulted in a reduction in gastric bilirubin exposure in 10 of 12 patients in group 2.
Group 1 and 2 patients combined: individual oesophageal acid reflux times before and during omeprazole treatment.
Group 1: individual oesophageal bilirubin reflux times before and during omeprazole treatment.
Group 2: individual gastric bilirubin reflux times before and during omeprazole treatment.
The two patients in whom gastric bilirubin exposure increased had the lowest gastric bilirubin exposure while off omeprazole. The reduction in gastric bilirubin exposure by omeprazole, although statistically significant, was not as extensive as the reduction in oesophageal bilirubin exposure.
Discussion
In addition to profound acid suppression, omeprazole has been shown to have a variety of other effects on upper gastrointestinal physiology. These effects in turn may influence DGR and DGOR. Omeprazole causes a rise in basal gastrin concentrations,18 ,19 pepsinogen 1 concentrations,18 and meal stimulated gastrin concentrations.20 Gastrin has been shown to decrease gastric emptying,21 ,22 so the hypergastrinaemia resulting from omeprazole treatment may theoretically prolong DGR. Indeed, several studies have shown omeprazole to slow gastric emptying in healthy subjects by a decrease in the solid emptying phase.23-25 A study in duodenal ulcer patients, however, showed omeprazole to have no effect on gastric emptying.26Conversely, omeprazole has been shown to augment the antral and duodenal phase III migrating motor complex in healthy controls, thereby cleansing the antroduodenal region more rapidly of secretions and theoretically reducing DGR.27 Thus the evidence seems to be conflicting, with the existence of mechanisms for potentially both increasing and reducing DGR. There has been little investigation of the effect of omeprazole on oesophageal motor function. Two studies, however, have shown omeprazole to have little effect on lower oesophageal sphincter pressure.28 ,29
The reduction in DGOR by omeprazole 20 mg twice daily seen in the present study is in keeping with an earlier report by Champion et al who observed a reduction in six of nine patients to below 7% total bilirubin exposure time.7 They proposed that this effect was secondary to the reduction in the volume of gastric secretions, with less fluid available in the stomach for any DGR to mix with and thence to reflux into the oesophagus. It known that omeprazole 20 mg twice daily reduces the volume of gastric secretion by about 40%,18 ,30 and this seems to be a plausible mechanism for the decrease in DGOR.
This mechanism may also explain the significant reduction in DGR to the upper stomach seen in the present study. By reducing the gastric volume available for duodenal refluxate to mix with, the refluxate may be less able to reach the upper stomach. The reduction in DGR, however, is less dramatic than the reduction in DGOR, as might be expected if this were the mechanism. Prior to treatment there was no significant difference between DGR and DGOR, suggesting that the lower oesophageal sphincter may have little effect in preventing duodenal contents refluxing into the oesophagus in patients with Barrett’s oesophagus. An alternative explanation is that bilirubin absorption is reduced by up to 30% in an acid milieu,10 which means that the bilirubin probe may be underestimating DGR. It must be remembered that the bilirubin probe is only detecting bilirubin in a single area of the stomach and this study says nothing, for instance, about the extent of DGR and effect of omeprazole on DGR in the antrum, which is the subject of future investigation. It does show, however, that the upper stomach is not continuously bathed in an alkaline duodenal refluxate in patients who are taking omeprazole. It is interesting to speculate whether DGR plays a role in the pathogenesis of carditis or intestinal metaplasia of the cardia, both of which are thought to be early signs of gastro-oesophageal reflux disease.31 Further investigation of gastric bilirubin exposure in health and disease is required before firm conclusions can be drawn.
An antireflux operation has the advantage of preventing both acid and duodenal content reflux into the oesophagus without necessitating long term acid suppression and the possible detrimental effects of this on the gastric mucosa.8 Despite this, probably the majority of patients with Barrett’s oesophagus are treated medically, either because they are symptomatically well on tablets, because they do not want an operation, or because they are not fit for surgery. Advances in endoscopic treatment of Barrett’s oesophagus (such as laser treatment or photodynamic therapy) mean that poor general health is not necessarily a bar to treatment, should high grade dysplasia or in situ carcinoma develop. It would therefore seem logical that medical treatment is effective at eliminating both components of reflux in these patients. It has been shown that symptomatic relief with omeprazole 20 mg twice daily does not necessarily mean elimination of acid reflux.32 Katzka et al argue that all Barrett’s patients should undergo 24 hour pH monitoring to ensure elimination of reflux.33 This is supported by the present study, in which two patients required higher doses of omeprazole to eliminate acid reflux (and DGOR in one case) despite being asymptomatic on omeprazole 20 mg twice daily. It seems sensible to prescribe a dose of omeprazole which eliminates pathological acid reflux, although the question of whether this should be extended to include the elimination of bilirubin reflux remains unanswered.
In summary, omeprazole significantly reduces reflux of both acid and duodenal contents into the oesophagus. In addition, the delivery of duodenal contents to the upper stomach is significantly reduced, although not to such a dramatic extent as in the oesophagus. This is encouraging for the large number of Barrett’s patients whose reflux disease is treated medically.
Acknowledgments
Part of this work was presented to the British Society of Gastroenterology, Manchester, September 1996, and published in abstract form (Gut 1996;39(suppl 1):A29.