Article Text
Abstract
Background: Six to 12 months of ingestion of moderate amounts of oats does not have a harmful effect in adult patients with coeliac disease. As the safety of long term intake of oats in coeliac patients is not known, we continued our previous 6–12 month study for five years.
Aim: To assess the safety of long term ingestion of oats in the diet of coeliac patients.
Patients: In our previous study, the effects of a gluten free diet and a gluten free diet including oats were compared in a randomised trial involving 92 adult patients with coeliac disease (45 in the oats group, 47 in the control group). After the initial phase of 6–12 months, patients in the oats group were allowed to eat oats freely in conjunction with an otherwise gluten free diet. After five years, 35 patients in the original oats group (23 still on an oats diet) and 28 in the control group on a conventional gluten free diet were examined.
Methods: Clinical and nutritional assessment, duodenal biopsies for conventional histopathology and histomorphometry, and measurement of antiendomysial, antireticulin, and antigliadin antibodies.
Results: There were no significant differences between controls and those patients consuming oats with respect to duodenal villous architecture, inflammatory cell infiltration of the duodenal mucosa, or antibody titres after five years of follow up. In both groups histological and histomorphometric indexes improved equally with time.
Conclusions: This study provides the first evidence of the long term safety of oats as part of a coeliac diet in adult patients with coeliac disease. It also appears that the majority of coeliac patients prefer oats in their diet.
- coeliac disease
- gluten free diet
- oats
- villous atrophy
- CD, coeliac disease
- ARA, antireticulin antibodies
- AGA, antigliadin antibodies
- EMA, antiendomysial antibodies
Statistics from Altmetric.com
- CD, coeliac disease
- ARA, antireticulin antibodies
- AGA, antigliadin antibodies
- EMA, antiendomysial antibodies
Since the pioneering work of Dicke1 published in 1950, the toxic effect of wheat and rye to coeliac patients has been known. Three years later, the same group suggested that oats was also harmful to patients with coeliac disease (CD).2 Corn and rice were found to be inert.3
Our previous 6–12 month study demonstrated no harmful effects of oats, as reflected by symptoms, nutritional status, duodenal villous architecture, and mucosal mononuclear cell infiltrate in coeliac patients in remission. In subjects with newly diagnosed disease, oats also did not delay recovery of mucosal damage.4 Subsequently, other short term investigations confirmed our results.5–,8 Recent results from our study also indicate that oats does not induce cellular or humoral immunological responses within 12 months in adults with CD.9 As the long term safety of oats has been questioned, we decided to re-examine patients from our previous study at five years. Patient assessments included clinical examination, nutritional status, duodenal mucosal histopathology and histomorphometry, and laboratory parameters, including antiendomysial (EMA), antireticulin (ARA), and antigliadin (AGA) antibodies.
METHODS
Patients
The inclusion and exclusion criteria for patients in the original oats intervention study have been described in detail previously.4 Two groups were originally examined: CD patients in remission (n=52) and newly diagnosed patients (n=40). Eleven patients withdrew from the original study: six from the remission group and five from the newly diagnosed group.
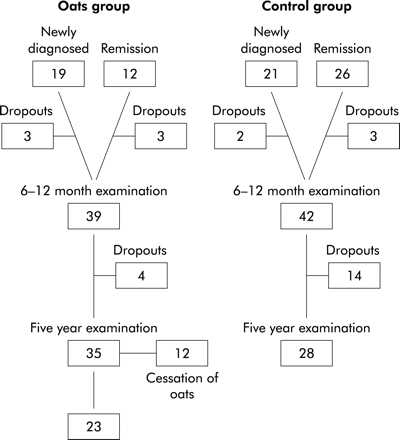
After the 6–12 month intervention study, patients in the oats group were given a choice of whether to continue or discontinue consuming oats. Those on a conventional diet were asked to continue their gluten free diet without oats. Five years after participation in the original intervention study, patients were invited for re-examination. All subjects were regarded as “old” coeliac patients because their diagnosis had been established at least five years earlier. At the five year examination they formed either an oats group or a control group. Thirty nine patients in the oats group and 42 in the conventional diet group (control) were contacted. Four patients from the oats group withdrew from the study without giving a reason. One patient in the control group withdrew because of pregnancy, two because of malignancy (breast cancer, parotid gland malignancy), and 11 had no specific reason for withdrawal. After five years, 23 of 35 subjects (65.7%) in the oats group still used oats. Those 12 subjects who shifted from an oats diet to a conventional gluten free diet reported that the main reason for discontinuing oats was doubt about its safety at that time. Figure 1⇓ shows the formation of the study groups.
Formation of the study groups for the five year examination.
Diets
Initially, a clinical nutritionist gave both verbal and written instructions on diet. The oats group received products supplemented with oats so that the goal of the daily intake of oats was 50–70 g. After the intervention study of 6–12 months duration, subjects belonging to the oats consuming group were allowed to continue taking oats freely. Oat products were not supplied free of charge but patients bought oat products (oats, rolled) from general stores. The purity of the oats and gluten free products was monitored during the 6–12 month intervention4 but after that there was no systematic monitoring. However, the oat products used in Finland have been found to be free of gluten contamination.9 Control patients were asked not to take oats but were recommended to continue on a strict conventional gluten free diet.
Examinations
After five years from starting the oats intervention study,4 upper gastrointestinal endoscopy was performed with an Olympus GIF Q20 end viewing gastroscope, and duodenal biopsies were obtained at the duodenal bulb and thereafter at 5 cm intervals as far as possible, two specimen at each level, using jumbo forceps (Olympus FB-13K). Altogether, 10 postbulbar biopsies per patient were obtained during the endoscopic procedure at the five year examination.
Methods of histopathological and histomorphometric analyses have been described in detail previously.4 The degree of crypt hyperplastic villous atrophy was divided into four classes: 0, normal; 1, partial atrophy; 2, subtotal; and 3, total villous atrophy. Mononuclear cell infiltration was graded as: 0, none; 1, mild; 2, moderate; and 3, severe. The length of the surface area of villi and crypts was measured and their ratio was calculated on three random fields from each level of the biopsy specimen. The level index was the mean of all three calculated ratios and the final index was the mean of all level indexes.4
IgA and IgG AGA were measured using an inhouse solid phase ELISA.10 ARA of class IgA and EMA of class IgA were determined by indirect immunofluorescence.11, 12 Normal levels of AGA IgA are 0.5 EU/ml and of AGA IgG <20 EU/ml; normal values of ARA IgA and EMA are titres <5.
Nutritional status and laboratory examinations were performed as in the original study.4 Adherence to diet, nutrient intake, and consumption of cereals were examined using a four day food record and compliance questionnaire.4
Informed consent
The study protocol was approved by the ethics committee of Kuopio University Hospital. All patients received written information concerning the trial, and verbal consent was obtained from each patient before starting the diet.
Statistical analysis
All analyses were carried out using both per protocol and intention to treat principles. All results in the text are presented based on the per protocol principle. In table 2⇓ results are presented using both principles. Statistical analyses were performed using the SPSS-PC (SPSS Inc., Chicago, Illinois, USA) and CIA programs.13 Non-parametric tests were used when variables were not normally distributed and did not have equal variance. Differences between groups were assessed using the Student's t test (two tailed t test) or the Mann-Whitney U test. Results are presented as mean (SD) or median (range). Fisher's exact test was used to analyse differences in the frequency between the study groups. The 95% confidence intervals were calculated for the mean difference in the changes between the two groups.
RESULTS
In the oats group there were 13 men and 22 women; in the control group there were 10 men and 18 women. Mean age of subjects in the oats group was 53 (12) years, and in the control group 52 (10) years. Time from diagnosis and thereafter the duration of the gluten free diet was 10 (7) years and 10 (6) years, respectively. There were no significant differences in body mass index or change in body mass index, nutritional status, or routine laboratory data between the two groups at the five year examination (data not shown).
Oats consumption and compliance
Of the original oats consuming group, 21 (80.8%) patients in remission and 14 (73.7%) newly diagnosed patients with CD used more than 30 g of oats per day at the end of the 6–12 month study. During the five year follow up, mean intake of oats in the oats group was 34 (10–70) g/day. Twenty patients (57.1%) ate oats at least twice a week and three (8.5%) once a week or occasionally, but when used they consumed large amounts. Compliance with the coeliac diet was good: 25 (71.4%) patients in the oats group and 22 (78.6%) patients in the control group followed a strict gluten free diet (table 1⇓). Patients in the oats group who had decided to interrupt oats ingestion before the five year examination reported the following reasons: uncertainly of the safety of oats, flatulence (one patient), and rash (two patients).
Frequency of oats intake and adherence to a gluten free diet
Histology, histomorphometry
At the end of the original trial, the mean grade for duodenal villous atrophy of those subjects participating in the five year follow up study was 0.75 (0.41) in the oats group and 0.69 (0.41) in the control group (table 2⇓). After five years there was an improvement in the villous architecture in both groups. The change from the values at the end of the 6–12 month intervention in the oats group was −0.55 (0.51) and that of the control group −0.52 (0.45). The difference in the changes between the groups was −0.2 (−0.41; 0.14) indicating equal improvement. The mean histomorphometric index was 0.021 (0.003) in the oats group and 0.020 (0.003) in the control group at the end of the original intervention study, and after five years 0.017 (0.003) in both groups (table 2⇓). Mononuclear cell infiltration showed a similar improvement in both groups: the change from the values at the end of the 6–12 month intervention was −0.45 (0.89) in the oats group and −0.58 (0.83) in the control group. The difference in the changes between the groups was 0.1 (−0.36; 0.62) (table 2⇓).
Histopathological and histomorphometric values* of duodenal biopsies at the end of the intervention study at 6–12 months and after five years, in the oats and control groups
Endomysial, antigliadin, and antireticulin antibodies
After five years there were no significant differences between the oats and control groups for the frequency of high values of ARA (2/23 and 2/28), AGA IgA (4/23 and 3/28) or AGA IgG (1/23 and 2/28), or EMA IgA (1/23 and 2/28), respectively. At the end of the original trial, the frequency of high values of ARA, AGA IgA, and AGA IgG of those subjects participating in the five year follow up study were 1/23, 2/23, and 0/23 in the oats group and 0/28, 2/28, and 0/28 in the control group. There were no significant differences in these antibodies between the oats and control groups at any time point in the study. Abnormal values in both groups were found in patients with poor adherence to the gluten free diet.
DISCUSSION
Recent studies utilising histological, histomorphometric, and immunological methods have suggested that both adults4–6, 9 and children7, 8 with CD can use oats safely as part of an otherwise gluten free diet. This is also valid for dermatitis herpetiformis.14, 15 Since the follow up period in all of these studies including ours4, 9 did not extend beyond 24 months, there was a need to examine the effects of longer term exposure.16, 17 Hence we extended our study to five years.
Our results confirm that ingestion of oats does not result in any duodenal mucosal damage in adult CD patients examined using histological, histomorphometric, and immunological methods. Interestingly, in the present study duodenal mucosa showed slow but definite improvement even after several years on a gluten free diet both with and without oats. Oats had no effect on laboratory parameters, including AGA, ARA, and EMA. The high antibody titres in some patients in both groups could be explained by poor compliance to a gluten free diet.
The reason why CD patients can tolerate oats must be based on structural differences of proteins among oats, wheat, barley, and rye. The injurious agent in wheat in CD is in the ethanol soluble fraction, the gliadins,2 whose toxicity remains after peptic-tryptic digestion.18 It is possible that the absence of certain amino acid sequences found in wheat gliadin, but not in oat avenin, make oats tolerable to coeliac patients.19 Indeed, purified avenin does not activate CD or dermatitis herpetiformis when taken over several days.20 Recent guidelines from the Finnish and UK Coeliac Societies conclude that moderate amounts of oats can be consumed by most coeliac patients without risk.
Compliance in maintaining a strict gluten free diet is low among young adult coeliac patients.21 Kumar et al found that only 44% of 102 young adult patients maintained a strict diet despite repeated recommendations.21
Removal of oats from the list of forbidden cereals in the coeliac diet could increase compliance with a gluten free diet by giving more choices and reducing the cost of gluten free foods. In our study, adherence to the diet was relatively high: 71% in the oats group and 82% in the control group adhered to a strict gluten free diet, and none of the patients changed to a normal diet. This result may reflect selection bias, the influence of regular follow ups, as well as interest in the diet by patients.
In conclusion, our results show that even long term use of moderate amounts of oats included in a gluten free diet in adult patients with CD is safe. If allowed, most patients with CD prefer some oats in their diet.
Acknowledgments
Supported by grants from the Yrjö Jahnsson Foundation and the Finnish Gastroenterological Association.
REFERENCES
Footnotes
↵† Present address: Department of General Medicine, Al Mafraq Hospital, Abu Dhabi, UAE
This study was performed in the Departments of Medicine and Clinical Nutrition, University of Kuopio and Kuopio University Hospital, Finland