Article Text
Abstract
Objective IBS is associated with an intestinal dysbiosis and faecal microbiota transplantation (FMT) has been hypothesised to have a positive effect in patients with IBS. We performed a randomised, double-blind placebo-controlled trial to investigate if FMT resulted in an altered gut microbiota and improvement in clinical outcome in patients with IBS.
Design We performed this study in 52 adult patients with moderate-to-severe IBS. At the screening visit, clinical history and symptoms were assessed and faecal samples were collected. Patients were randomised to FMT or placebo capsules for 12 days and followed for 6 months. Study visits were performed at baseline, 1, 3 and 6 months, where patients were asked to register their symptoms using the IBS-severity scoring system (IBS-SSS) and IBS-specific quality of life (IBS-QoL). Prior to each visit, faecal samples were collected.
Results A significant difference in improvement in IBS-SSS score was observed 3 months after treatment (p=0.012) favouring placebo. This was similar for IBS-QoL data after 3 months (p=0.003) favouring placebo. Patients receiving FMT capsules had an increase in faecal microbial biodiversity while placebos did not.
Conclusion In this randomised double-blinded placebo-controlled study, we found that FMT changed gut microbiota in patients with IBS. But patients in the placebo group experienced greater symptom relief compared with the FMT group after 3 months. Altering the gut microbiota is not enough to obtain clinical improvement in IBS. However, different study designs and larger studies are required to examine the role of FMT in IBS.
Trial registration number NCT02788071.
- irritable bowel syndrome
- colonic microflora
Statistics from Altmetric.com
Significance of this study
What is already known on this subject?
The gut microbiota in some subgroups of patients with IBS is different from healthy controls.
In Clostridium difficile infections, faecal microbiota transplantation (FMT) has shown excellent effects.
What are the new findings?
This is the first large randomised controlled trial assessing the efficacy and safety of FMT in IBS with data on changes in the gut microbiota.
FMT can change the gut microbiota in patients with IBS, but has less effect on symptoms and quality of life compared with placebo.
How might it impact on clinical practice in the foreseeable future?
This study does not show evidence for effective treatment of IBS with FMT, despite long-term changes in gut microbiota after FMT was shown in these patients.
More research regarding the effect of FMT in IBS subgroups is needed before the real impact of FMT in IBS is known.
Introduction: background and objectives
IBS is the most commonly diagnosed GI condition, and affects up to one in five people at some point during their lives.1 In accordance with the Rome III criteria,2 IBS is characterised by abdominal discomfort or abdominal pain and altered bowel function, without alarm symptoms such as blood in stools and weight loss. IBS can be further categorised into diarrhoea-predominant, constipation-predominant or mixed type.1
Many theories have been put forward but the exact cause of IBS is still uncertain. The complexity and diversity of IBS presentation makes treatment difficult.
Current evidence suggests that the microbiota of the GI tract could be a significant factor in the aetiology of IBS.3 Changes in the intestinal environment are hypothesised to induce a compositional imbalance of the gut microbiota, termed ‘dysbiosis’, which is associated with IBS.4 Several studies have demonstrated that the composition of the gut microbiota in patients with IBS is different from healthy controls.5
Worldwide, interest in faecal microbiota transplantation (FMT) as an ‘ecological’ therapy for several diseases, including IBS, is growing rapidly. Human faeces from healthy donors contain more than a 100 different types of bacteria, along with parasites, viruses, fungi and bacteriophages, which may also play a significant role. In recurrent Clostridium difficile infections, FMT has shown excellent effects. FMT has a much higher cure rate than standard treatment6 and studies have shown that FMT might restore intestinal microbial balance in treated patients.7–11 FMT has, in smaller studies, been shown to be able to create lasting changes in the colonic microbiota, which can be detected up to 6 months after the treatment.12
FMT could therefore theoretically be a possible treatment for patients with IBS.
To date, one randomised placebo-controlled study and only few other smaller, non-randomised placebo-controlled studies have evaluated whether FMT is effective in patients with IBS.13 14 To clarify the effects of FMT on symptoms and gut microbiota in patients with IBS, we performed this randomised, double-blind placebo-controlled pilot study.
Methods
Trial design
Patients were included in a 6-month randomised double-blind placebo-controlled study and allocated to treatment with FMT capsules or placebo capsules. Twenty-five capsules were consumed while fasting every morning for 12 days. Before the first treatment, the participants had a bowel cleansing with Picoprep performed corresponding to the procedure before a colonoscopy.
All patients were seen for study visits at baseline, 1, 3 and 6 months, where they completed the IBS-severity scoring system (IBS-SSS)15 and IBS-specific quality of life (IBS-QoL).16 17 Additionally, patients were asked to keep a daily diary including Bristol Stool Form Scale,18 symptoms, use of laxatives and side effects of the treatment, if any.
The primary end point was to evaluate the reduction of IBS-SSS in the treatment group compared with the placebo group at 3 months. Secondarily, we evaluated change in IBS-QoL scores at 3 months and changes in microbiota diversity before and after FMT.
Participants
Adult patients with IBS (aged 18–60 years) were recruited between October 2016 and December 2016 from the Department of Gastroenterology, Aleris-Hamlet Hospitals Copenhagen, Søborg, Denmark and Department of Gastroenterology, Copenhagen University Hospital Hvidovre, Copenhagen, Denmark. Patients were diagnosed with IBS according to Rome III criteria.2
In addition, criteria were as follows:
Inclusion criteria:
Moderate-to-severe disease activity (IBS-SSS ≥175);
Able to read and speak Danish;
Normal colonoscopy (performed within 1 year) if the patient was ≥40 years or if the patient had blood in stool.
Exclusion criteria:
Other chronic GI disease;
Faecal sample positive for enteropathogenic microorganisms;
Positive screening for HIV, HBV or HCV antibody;
Surgical interventions in the GI region (except for appendectomy, hernia repair, cholecystectomy and gynaecological and urological procedures);
Psychiatric disorder;
Faecal calprotectin ≥50 mg/kg;
Abuse of alcohol or drugs;
Medications other than birth control pills, hormone supplements, allergies/asthma agents, blood pressure and cholesterol-lowering agents, proton pump inhibitors and non-prescription medicines;
Abnormal screening biochemistry;
Abnormal colonoscopy findings;
Pregnant, planned pregnancy or breastfeeding females;
Ingestion of probiotics or antibiotics <8 weeks before the inclusion.
Furthermore, all patients were subclassified in three different IBS subtypes: constipation-predominant (IBS-C), diarrhoea-predominant (IBS-D) or alternating periods of constipation and diarrhoea (IBS-M).18 Demographic information was obtained in all participants and they reported their current use of medications and completed questionnaires to characterise their symptom severity and bowel habits. The patients were asked to live as they used to through the half year study period.
Only a few patients fulfilling inclusion criteria declined participation.
Donors
Four faecal donors were recruited to this study. Once recruited, the donors were instructed to keep up a healthy lifestyle during the collecting period. They were all screened according to guidelines19 20 and were recruited according to the following criteria:
Inclusion criteria:
Aged between 18 and 45 years;
Previously and currently healthy;
Normal weight (body mass index (BMI) between 18.5 and 24.9 kg/m2);
Normal bowel movements (defined as 1–2 per day and type 3–4 at Bristol Stool Form Scale);
No medication consumption.
Exclusion criteria:
Known or high risk of infectious diseases such as HIV, HAV, HBV or HCV;
Positive stool sample for C. difficile toxin, parasites or other enteropathogens;
Antibiotic treatment in the past 6 months;
Abuse of alcohol or drugs;
Smoking;
Tattoo or body piercing within the last 6 months;
Allergy, asthma or eczema;
Family history of GI diseases, cancer, diabetes, obesity, autoimmune diseases, allergy, asthma, eczema, cardiovascular diseases, neurologic or mental illnesses;
Participation in high-risk sexual behaviours;
Born by caesarean section.
Sample preparation
Donors were equipped with 500 mL bottles of oxygen reduced sterile saline (0.9% NaCl), which they were instructed to keep refrigerated. Immediately after producing the sample, donors were instructed to cover the sample in the oxygen reduced saline to protect the sample from oxygen and to deliver it to our facility within 1 hour. Thereafter, the sample was stored at 5°C and processed in the laboratory no more than 3 hours later from the time of delivery. Samples with saline were homogenised manually, using a 400 mL BagPage XR from Interscience with a 250 µm filter to remove undigested fibrous material and centrifuged at 3000x g for 20 min at room temperature to remove the added saline. In this way we obtained a more concentrated product. The supernatant was discarded and the pellet was mixed with glycerol as a cryoprotectant to a final concentration of 30% glycerol. The effect of glycerol 30% in the FMT capsules was for freeze protection. With a lower amount of glycerol our capsules dissolved at −20°C. Most of the process was done in an anaerobic environment, using Argon gas to protect the sample. Faecal matter was briefly exposed to oxygen only while being transferred between containers. Finally, the samples were frozen at −20°C. Once donors had passed the second screening, all faecal samples were mixed into one batch for the entire experiment before being double encapsulated using Capsugel DR Caps size 0 and 00. One daily dosage of 25 such capsules contains approximately 12 g of the frozen faecal matter, which was derived from approximately 50 g of fresh faeces.
Interventions
FMT were in capsule form, packaged in plastic bottles. FMT and placebo capsules were identical in appearance: form, colour and size. Placebo capsules where made from saline, glycerol and food colouring E150. Also the placebo contained 30% glycerol. The identity of the capsules was unknown to participants, researchers and primary investigators. Participants were instructed to consume orally 25 capsules per day in the morning with water. It was allowed to eat an hour after capsule intake to ensure that most of the capsules had passed the stomach. The first daily dose was taken under supervision at the Department of Gastroenterology, Aleris-Hamlet Hospitals Copenhagen, where the study visits took place.
Faecal sample collection
Faecal samples were longitudinally collected from patients at baseline before bowel cleansing, 3 days after FMT treatment had stopped, and 1, 3 and 6 months after inclusion. The collected fresh faeces were stored in RNA later by the patients and brought to the hospital at study visits.
Library preparation and sequencing
Total DNA was extracted from 0.25 g of faecal samples using a PowerLyzer PowerSoil DNA Isolation Kit (MoBio 12 855–50) according to the manufacturer’s instructions. The V3-V4 hypervariable regions of 16S rRNA genes were amplified using primers 341F and 806R and indexed Illumina compatible 16S amplicon libraries were prepared as described elsewhere.21 Paired end sequencing (2×301 bp) was performed on the Illumina MiSeq platform with the MiSeq Reagent kit V.3.
Sequence processing and microbiomics analysis
Sequencing reads were demultiplexed using bcl2fastq V.2.17.1.14 (Illumina). Primer sequences were striped from the 5’ ends of each read in a pair and reads without discernible primer sequences were discarded using a custom Biopython script. Reads were processed with the UPARSE pipeline using usearch V.10.0.240_i86linux32 to generate an operational taxonomic unit (OTU) table at 97% granularity.22 The UPARSE-pipeline was modified, replacing the ‘read quality filtering’ and ‘length trimming’ steps with an alternative quality filtering (usearch -fastq_filter -maxee 1.0).23
Usearch was also used to construct an OTU tree and assign taxonomy to the OTUs’ centroids24 25 using the ribosomal database project reference 16S training set with species names (V.16) as a reference database. Taxonomy assignments were added to the OTU table with a custom Python script and the OTU table with taxonomy information was converted into biom format using biom.26 Diversity metrics were calculated using the Qiime V.1.9.1 core_diversity_analyses.py27 pipeline using a sampling depth of 5000 and default parameters otherwise.
OTU abundances in pairs of samples were assessed for significant differences using the Mann-Whitney U test, corrected for multiple sampling with the Benjamini-Hochberg method (FDR=5%). Correlations between IBS-SSS and OTU abundances and correlations between IBS-SSS and α-diversity were assessed using Spearman-Rank correlation analysis, corrected for multiple sampling with the Benjamini-Hochberg method (FDR=5%). Significant differences in α-diversity (chao1 metric) between treatment groups were assessed using the Mann-Whitney U test (p≤0.05). Differences in β-diversity distances were assessed using a Mann-Whitney U test (p≤0.01). SourceTracker was used to infer the proportions of microbial communities that come from possible source environments.28
Ethics
The study was performed in accordance with the requirements of Good Clinical Practice and the Revised Declaration of Helsinki. The study was registered in www.clinicaltrials.gov (NCT02788071). All participants provided written informed consent to participate after verbal and written information about the study. Participants could discontinue at any time point on their request. As FMT, according to the Danish Health Authority, is not considered as a pharmaceutical, no authorisation by the Danish Medicines Agency was required. All authors had access to the study data and reviewed and approved the final manuscript.
Outcomes
Sample size
We aimed to include 52 participants based on an effect of placebo of 0.40 and an effect of FMT of 0.80, with a power (1-β) of 0.80 and an alpha of 0.05 (two-tailed test) for intention-to-treat analysis.
Sample size was estimated assuming that 40% and 80% of patients in the placebo and FMT group, respectively would achieve the primary end point criteria at 3 months after inclusion.
Randomisation
Patients were randomised 1:1 to FMT capsules or placebo capsules and included by consecutive numbers. The randomisation was done in blocks of 4 by a researcher, not involved in the patients’ treatment and was generated by using the website Randomization.com ⟨http://www.randomization.com⟩. Investigators, patients and outcome assessors were kept masked to the allocation and intervention. The randomisation key was revealed to the researchers when participants completed the 6-month follow-up and data analysis was completed.
Statistical methods
All statistical analyses were done in RStudio (RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Boston, Massachusetts, USA; http://www.rstudio.com/). The number, percentage, mean, SD, mean difference and 95% CIs were reported. The IBS-QoL score was transformed into a 0–100 scale using the formula: total score=(sum of the items−34/170)×100. Student’s t-test was used to determine the difference between placebo and FMT. A paired t-test and a repeated measure analysis of variance (rm-ANOVA), was used to determine, within each group, the difference between inclusion and 1, 3 and 6 months follow-up. All tests were done for both IBS-SSS and IBS-QoL. Spearman’s test was used to determine the correlation between IBS-SSS and IBS-QoL. A score between 0 and 0.19 was interpreted as very weak, 0.20 and 0.39 as weak, 0.40 and 0.59 as moderate, 0.60 and 0.79 as strong and 0.8 and 1 as very strong.
Two-way ANOVA was used to determine factors associated with decrease in either IBS-SSS or IBS-QoL score. If interaction was found between variates, a linear regression model was used instead, controlled by quantile-quantile plot of residuals.
Furthermore, a cut-off of 50 points15 reduction after 3 months in the IBS-SSS was used to distinguish between ‘effect’ and ‘no effect’. This end point was analysed using the χ2 test. A logistic regression was used to determine associated factors for ‘effect’. Age, gender, BMI, previous or concurrently used IBS medical therapy, birth by caesarean section, breast feeding, IBS subtype, previous attempt with change in diet and weight loss were used as independent variables.
Measures
IBS disease severity was measured by using the IBS-SSS questionnaire,15 which includes five items on a 0–100 mm visual analogue scale with total score ranging from 0 to 500 mm. Question 1: severity of abdominal pain, question 2: frequency of abdominal pain, question 3: severity of abdominal distension, question 4: dissatisfaction with bowel habits and question 5: interference with quality of life.
Alterations in quality of life during the study were measured by IBS-QoL questionnaire, which consists of 34 items, each with a 5-point response scale. The 34 items are based on the following eight variables: health worries, food avoidance, body image, dysphoria, interference with activity, social reactions, sexual activity and relationships.17
Results
Patient characteristics
Overall, 52 patients were included in the study and randomised into two groups of 26 patients.
Patients in the two groups were comparable (table 1).
Baseline characteristics of patients
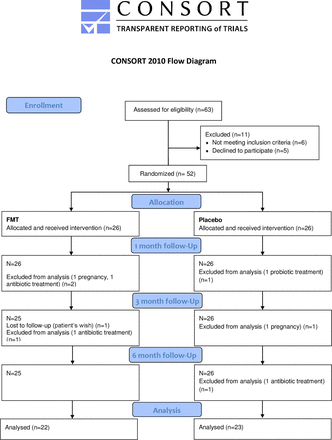
Only one patient dropped out while in the study, with no reason given. Other participants were excluded from the analysis due to the exclusion criteria (figure 1).
Flow chart of patients included and excluded from analysis. CONSORT, Consolidated Standards of Reporting Trials; FMT, faecal microbiota transplantation.
When participants were asked whether they were treated with FMT or placebo, only 25 patients guessed correctly (14 correctly guessed placebo and 11 correctly guessed FMT).
Disease severity (IBS-SSS)
The primary end point showing a significant difference in improvement between inclusion and the 3-month visit (mean (SD), FMT vs placebo; −52.45 (97.72) vs −125.71 (90.85), p=0.012) favouring the placebo group (table 2 and figure 2). The IBS-SSS score difference and score change over time at inclusion, 1, 3 and 6 months between placebo and FMT are shown in table 2 and figure 2.
IBS-SSS and IBD-QoL score between groups and their change over time
IBS-SSS score and IBS-QoL score between groups and their change over time. FMT, faecal microbiota transplantation; IBS-QoL, IBS-specific quality of life; IBS-SSS, IBS-severity scoring system.
The differences in scores between inclusion and 1, 3 and 6 months within each group were all statistically significant (data shown in online supplementary) and rm-ANOVA; FMT: p<0.01, placebo: p<0.01. No associated factors were found in the linear regression model or in the logistic regression model for a score change of 50 and above.
Supplemental material
Eight (36.4%) of 22 patients receiving FMT capsules vs 19 (79.2%) of 24 receiving placebo capsules showed a decrease in IBS-SSS of more or equal to 50 point at 3 months after inclusion (p=0.008).
Subgroup analysis in the different IBS types (IBS-C, IBS-D and IBS-M) at the 3 months visit showed higher improvement in IBS-SSS in the placebo group in all subgroups; however, a statistically significant effects was only seen among patients with IBS-C; mean (SD) FMT 42.2 (40.07) and placebo 149.12 (85.4) (p=0.025) (see online supplemental material table 2).
Supplemental material
Quality of life (IBS-QoL)
The IBS-QoL score difference and score change over time between placebo and FMT are shown in table 2 and figure 2; showing a significant difference in improvement between FMT and placebo at 3 months (mean (SD) FMT vs placebo; −7.22 (10.12) vs −16.50 (9.60), p=0.003) favouring the placebo group.
Likewise, the placebo group has a significantly better change in IBS-QoL score at 1 and 6 months compared with the FMT group. The improvements within FMT and placebo groups (separately) were all significant by both paired t-test (data shown in online supplemental material table 1) and rm-ANOVA; FMT: p<0.01, placebo: p<0.01. No associated factors were found in the ANOVA.
The correlation between IBS-SSS and IBS-QoL was found to be 0.71 by Spearman’s correlation coefficient and therefore strong.
Side effects
The majority of the patients experienced side effects (22 (84.6%) in the FMT group and 15 (57.7%) in the placebo group (table 3).
Side effects in the FMT and placebo group.
There were no side effects that were more prominent in the FMT group compared with placebo except diarrhoea (p=0.03), experienced by the patients during the FMT treatment period. No serious adverse events were reported.
Microbiome analysis
A total of 8.3 million MiSeq paired reads were obtained, of which 7.7 million were classified into 2747 OTUs. Analysis of α-diversity showed that (i) faecal donors had higher microbiome biodiversity than patients with IBS (FMT and placebo groups separately) at inclusion, (ii) patients receiving FMT capsules had an increase in biodiversity to the extent that this group was not statistically distinguishable from the donors and (iii) the placebo patients remained statistically indistinguishable from their pretreatment state (Mann-Whitney U test, p<0.05, figure 3). In patients with IBS (FMT, placebo and at inclusion groups), α-diversity did not correlate with IBS-SSS (Spearman’s rank correlation test; R=−0.050, p=0.442). Even while assessing subgroups of the data (IBS-C, IBS-D or IBS-M, and FMT or placebo), there were no correlations between IBS-SSS and α-diversity.
α-Diversities of donors, FMT patients at inclusion (Pre FMT), placebo patients at inclusion (Pre Placebo) and FMT patients (FMT) and placebo patients (Placebo). Red lines represent median α-diversities (chao1 metric), the boxes range from the lower to the upper quartiles, the whiskers extend 1.5 times beyond the IQR and outliers are plotted in. The letters (a, b and c) above the median lines delimit the samples into statistically distinguishable groups based on the Mann-Whitney U test (p≤0.05).
Source tracking software28 inferred that a larger proportion of the FMT groups’ microbiotas originated from the FMT donors’ microbiotas in contrast with the placebo group (p<0.01 for all time points) (see online supplemental material figure 1). Correspondingly, a much larger proportion of the placebo groups’ microbiotas, in comparison with the FMT groups’ microbiotas, were inferred to have originated from the patients’ own microbiotas at initial assessment (p<0.01 for all time points) (see online supplemental material figure 1).
Supplemental material
Analysis of β-diversity (unweighted UniFrac) ordinated using principal coordinate analysis showed that the donors grouped close together at the edge of the ‘cloud’ of the microbiotas of patients with IBS at inclusion figure 4A, figure 4B. At all time points, the FMT recipients’ microbiotas had a higher density in the vicinity of the donors’ microbiotas (figure 4C), while the placebo recipients’ microbiotas, also at all time points, were dispersed over the cloud of the patients at inclusion (figure 4D). Statistical analysis of unweighted UniFrac pairwise distances (p<0.01) confirmed that FMT recipients’ microbiotas are more similar to the donors’ microbiotas than to the placebo recipients’ microbiotas. Furthermore, the placebo recipients’ microbiotas did not become more similar to the donors’ microbiotas than patients with IBS before randomisation. This confirms that treatment with FMT capsules caused the recipients’ microbiotas to more closely resemble the donors’ microbiotas.
Principal coordinate analysis-ordinated β-diversity plots (unweighted UniFrac distances) show that FMT capsules make the FMT group’s microbiotas look more similar to the donors’ microbiotas. (A) The pretreatment microbiotas of patients with IBS are dispersed in a ‘cloud’. (B) The healthy donors group together around the bottom left of the IBS cloud of patients with IBS. (C) The post-treatment FMT groups’ microbiotas are dispersed throughout the cloud of patients with IBS, but show a higher density near the donors' microbiotas. (D) The post-treatment placebo groups’ microbiotas are dispersed evenly around the pretreatment cloud. The first three principal axes are presented, with PC1 (horizontal) explaining 10.37%, PC2 (coming out of page) explaining 7.19% and PC3 (vertical) explaining 4.56% of the variation. The four individual donors’ microbiotas and two mixtures of the donors’ microbiotas are represented by the six points labelled as ‘donor’ in the legend, while statistical tests comparing donors with other groups used only data from the four individual donors for comparisons.
Analysis of OTUs between donors, patients at inclusion, patients 3 months after FMT treatment and patients 3 months after placebo treatment, revealed that 11 OTUs established in FMT recipients (see online supplemental material table 3). The criteria for this depended on the donors having significantly more of the OTU than the patients at inclusion, the FMT-treated patients having significantly more of the OTU than the patients at inclusion and the placebo patients not having significantly different levels of the OTU to the patients at inclusion. By corresponding criteria, we showed that no OTUs established ‘from donors’ in the placebo patients, indicating a high-likelihood that the OTUs seen to establish in the FMT patients originated from the FMT capsules and not from other natural means of acquiring new intestinal microbes. Of these 11 OTUs, 6 were classified in the Clostridiales order and 4 in the Bacteroidales order.
Supplemental material
Two OTUs had weak (0.16≤R<0.30) negative correlations with IBS-SSS, one OTU had a weak positive correlation with IBS-SSS and two OTUs had moderate (0.3≤R<0.5) positive correlations with IBS-SSS. None of these OTUs showed any significant changes in abundance due to FMT treatment. The two OTUs correlating negatively with IBS-SSS were both classified in the Blautia genus of the Clostridiales order which is associated with a healthy gut-microbiome.29–31 Of the three OTUs with positive correlations with IBS score, one was classified in the Bacteroides genus and two were classified in the Ruminococcaceae family. Neither of these groups has any particularly negative associations with health as they are extremely common inhabitants of the healthy human GI tract.
Discussion
In this study, we tested the use of FMT in 52 patients with IBS and characterised their faecal microbiotas before and after the treatment. Previously, FMT has been reported to reduce IBS symptoms in smaller not blinded studies13 and one RCT.14 Our study, however, is one of the first randomised double-blind placebo-controlled study to evaluate the efficacy of orally administered FMT capsules in patients with IBS with moderate to severe symptoms. No other RCT has either examined the composition of gut microbiota in donors and patients and the change in microbiota composition before and after FMT in patients with IBS.
Overall, a reduction of IBS symptoms was found in both FMT and the placebo group, but our results show a significantly better effect of placebo compared with FMT treatment on improvement in IBS-SSS after 3 months, which was our primary end point. Likewise, the improvements in IBS-QoL scores were significantly better in the placebo group 1, 3 and 6 months after inclusion.
The microbiome results showed that our patients with IBS had lower stool microbial biodiversity than the healthy donors (figure 3) and in an ordinated β-diversity analysis, the donors’ microbiotas had a ‘cloud’ on the periphery of a comparatively larger cloud of patients with IBS (pretreatment) (figure 4B). These observations support the view3 that there is a relationship of unknown causality between IBS and the intestinal microbiota. The consumption of FMT capsules was associated with an increase in stool microbial biodiversity above that seen in the placebo control group and the FMT capsules also resulted in the establishment of several OTUs in the recipients that did not establish in the placebo group. Ordination of β-diversity indicated that microbiotas of patients with IBS resembled donors’ microbiotas more closely following FMT capsule treatment and a microbiota source tracking software indicated that the FMT capsules brought about a long-term (at least 6 months) establishment of donor microbes in the recipients. These observations, in addition to the increase in biodiversity and the establishment of OTUs in the FMT treatment group, clearly demonstrate that the FMT capsules are having a lasting effect on the recipients’ microbiotas. However, these changes to the microbiotas did not improve the clinical prognoses of the recipients in any way. The low number of OTUs that had any correlation, positive or negative, with IBS score and the complete lack of correlation between α-diversity and IBS score indicate that there is no simple characteristic of the intestinal microbiota playing a role in IBS.
The strength of this study is that the patients could not guess what treatment they received, which is proof that the blinding was sufficient. None of our patients experienced serious adverse events. For this reason, we find the procedure feasible and safe in patients with GI symptoms.
However, limitations need to be taken into consideration. One such limitation could be that we included all IBS subtypes. The study by Johnsen et al 14 focused on IBS-D and IBS-M. The study showed that FMT induced significant symptom relief in patients with IBS, but no data on changes in the microbiota were presented. Nevertheless, subgroup analyses in our study indicate that the IBS-D subgroup did not do better than the other subgroups. We used a donor-mix of FMT were Johnsen et al used single donor FMT. This could also influence the results. Perhaps donor mix is not preferable. In the review of previous smaller studies of FMT in IBS, an improvement was described in 74% of participating patients with IBS.13 This impressive result could possibly be explained by inclusion of only specific IBS subtypes (such as IBS-D) in some of these studies. However, all studies reviewed were without placebo control, making the improvement rate of 74% less reliable and difficult to compare both with our study and the study by Johnsen et al. 14 Our capsules contained glycerol and we cannot exclude that glycerol had influenced bowel physiology and could have affected the outcome of the study. But both the FMT and placebo capsules contained glycerol.
Although our study shows a better effect of a bowel cleansing than a bowel cleansing followed by a FMT treatment on IBS symptoms, we still believe that FMT can be the way forward. Maybe the treatment should be approached in a different way. Could FMT counteract a possible positive effect of bowel cleansing? Maybe certain IBS harmful bacteria or other microbes could be lost during bowel cleansing and then reintroduced by FMT? Or should an antimicrobial therapy be used in advance of FMT treatment?
Several other factors could influence the effect of FMT, such as the route used for FMT, duration of treatment and quantity of faecal microbiota transplanted to the patient. No clinical trials have compared FMT delivery routes in IBS and further trials are needed to determine if a change in delivery route could have an effect on FMT treatment success in these patients.5 Also the use of FMT donor-mix versus single donor should be explored further.
There is no evidence for FMT providing symptomatic benefit in patients with IBS in this trial. No significant side effects to FMT were observed during 6 months follow-up.
Conclusion
In this randomised double-blinded placebo-controlled study, we found that FMT changed gut microbiotas in patients with IBS. But patients in the placebo group experienced greater symptom improvements compared with the FMT group after 3 months (p=0.012). However, more studies are required to examine the potential role of FMT in treating IBS.
Supplemental material
Acknowledgments
The authors would like to thank Thomas Kallemose for statistical assistance and Tina Thane and Tanja Begovic for technical assistance with DNA sequencing.
References
Footnotes
Contributors SIH, SG, AHC, LHH and AMP designed the research project; SG designed and produced the capsules, SIH and AHC performed the clinical research; BZSL performed the statistical analysis, LHH and PDB performed the sequencing and microbiota analysis, SIH, BZSL and PDB wrote the paper; AHC and AMP supervised the paper; all authors read, commented and approved the final manuscript.
Funding The study is financed by grants from the private foundations: Wedell-Wedellsborgs Fund, Toyota-Fonden Denmark, Danish Colitis-Crohn’s Associations Research Fund, Tømmerhandler Johannes Fogs Fond, Villy Safft Nielsens Fond, Villum Foundation Block Stipend, MicroHealth (Innovation Fund Denmark) and co-financed by Aleris-Hamlet Research and Development Fund and Department of Gastroenterology Hvidovre University Hospital.
Disclaimer The funding sources had no role in the study design, data collection, interpretation of analysis, writing of the manuscript or decision to submit the publication.
Competing interests None declared.
Patient consent Obtained.
Ethics approval This study was approved by the Ethical Committee, Denmark (protocol number: H-15016343).
Provenance and peer review Not commissioned; externally peer reviewed.
Correction notice This article has been corrected since it published Online First. Affiliation 3 has been corrected.