Article Text
Statistics from Altmetric.com
GI symptoms such as diarrhoea, nausea and vomiting are frequent coronavirus disease (COVID-19) symptoms and affect up to 28% of patients.1–5 The pathophysiology of COVID-19-associated GI symptoms is currently unclear. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-RNA was detected in the faeces in ~50% of patients with COVID-193 5 6; SARS-CoV-2 viral particles were observed by electron microscopy in stool samples from two patients without diarrhoea2; and one study reported SARS-CoV-2 infection of the oesophagus, stomach, duodenum and rectum.5
Faecal calprotectin (FC) has evolved as a reliable faecal biomarker allowing detection of intestinal inflammation in IBD and infectious colitis.7 In this pilot study, we explored a relation between GI symptoms, intestinal inflammation (determined by FC) and faecal SARS-CoV-2-RNA in hospitalised patients with COVID-19 who did not require intensive care measures.
We analysed 40 patients with COVID-19 hospitalised at the University Hospital of Innsbruck, Austria. Confirmation of SARS-CoV-2 infection was performed by nasopharyngeal swab, and faecal SARS-CoV-2-RNA detection was performed using previously described real-time PCR6 as recommended by the Centers for Disease Control and Prevention (DeKalb, Georgia). Diarrhoea was defined as loose stools >3 times/day. We excluded other causes of acute GI infection by stool analysis for common viral, bacterial, parasitic and protozoan pathogens in all patients with diarrhoea, and no other chronic intestinal disease was documented for any patient. FC concentration was determined by the Calprest ELISA (Eurospital, Trieste, Italy) according to the manufacturer’s specification. The lower detection limit of the assay is 16 µg/g. Faecal RNA isolation (for SARS-CoV-2 PCR) was performed using RNeasy PowerMicrobiome Kit (Qiagen, Venlo, The Netherlands) according to the manufacturer’s specifications. Data were expressed as mean±SEM. Statistical significance, determined by one-way analysis of variance or Spearman’s rank correlation in GraphPad Prism, was assumed at p<0.05.
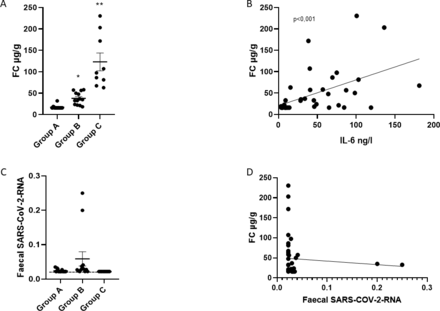
The characteristics, frequency of GI symptoms and biochemical parameters of 40 hospitalised patients with COVID-19 are listed in table 1. In this cohort, 18 (45%) patients did not report diarrhoea (group A), while 22 patients (55%) reported diarrhoea. We separated these patients into two groups because in 13 patients diarrhoea had ceased (>48 hours) before we collected the faecal samples (group B), while 9 patients still reported diarrhoea (symptom onset <48 hours) (group C). COVID-19 patients with ceased diarrhoea (group B) and to a larger extent patients with ongoing diarrhoea (group C) displayed elevated FC concentrations when compared with COVID-19 patients without diarrhoea (group A) (figure 1A, p<0.001). FC concentration significantly correlated with serum interleukin-6 (IL-6) concentration (figure 1B, p<0.001), but not C reactive protein (CRP) or ferritin (data not shown). SARS-CoV-2-RNA was detected in stools from 12 of 40 (30%) patients with COVID-19 (figure 1C). Notably, SARS-CoV-2-RNA was not detected in stools from patients with ongoing diarrhoea (group C), but in eight patients with ceased diarrhoea (group B) and in four patients without diarrhoea (group A). In line with this, no relation between faecal SARS-CoV-2-RNA and FC, IL-6, CRP or ferritin was noted (figure 1D; data not shown).
Increased FC and serum IL-6 feature acute diarrhoea in COVID-19. (A) FC concentration determined by ELISA. Group A (n=18): COVID-19 patients without diarrhoea. Group B (n=13): COVID-19 patients who reported diarrhoea which ceased 48 hours before stool collection. Group C (n=9): COVID-19 patients with acute diarrhoea (onset <48 hours) (*p<0.001, group B versus group A; **p<0.001, group C versus groups B and A). (B) Correlation of serum IL-6 concentration with FC concentration reported in A. (C) Detection of faecal SARS-CoV-2-RNA by PCR, reported as 1/cycle threshold value. Dashed line indicates threshold of RNA detection. (D) Correlation of faecal SARS-CoV-2-RNA (as in C) with FC. COVID-19, coronavirus disease 2019; FC, faecal calprotectin; IL-6, interleukin-6; SARS-CoV-2, severe acute respiratory syndrome corona virus 2.
Patient characteristics and biochemical parameters
Previous studies indicated that SARS-CoV-2 binds to cells in the GI tract (eg, small and large intestinal epithelial cells), likely via specific receptors such as ACE2 and the transmembrane serine protease 2.8 9 It is conceived that this virus infects epithelial cells causing cytokine and chemokine release, instigating acute intestinal inflammation characterised by infiltration of neutrophils, macrophages and T cells. We report evidence that SARS-CoV-2 infection in patients with COVID-19 indeed instigates an inflammatory response in the gut, as evidenced by diarrhoea, elevated FC (largely expressed by neutrophil granulocytes7) and a systemic IL-6 response. Faecal SARS-CoV-2 RNA was not detected during acute diarrhoea but could be detected in asymptomatic patients with or without previous diarrhoeal symptoms. It is currently unknown if SARS-CoV-2 infection affects the course of patients with IBD and whether immunosuppressive treatment affects their susceptibility to (or the course of) COVID-19.10 Our data support the notion that SARS-CoV-2 infection exerts gut tropism characterised by an acute inflammatory response that potentially deteriorates the course of human IBD.
Footnotes
Contributors ME, FG, TA and HT wrote the letter. LM, JS, SS, MN, SS-B, RH, MS and TM performed the laboratory analyses. GF, RB-W and GW were involved in patient care and contributed to the preparation of the manuscript.
Funding This study is supported by the excellence initiative VASCage (Centre for Promoting Vascular Health in the Ageing Community), an R&D K-Centre (COMET programme - Competence Centers for Excellent Technologies) funded by the Austrian Ministry for Transport, Innovation and Technology, the Austrian Ministry for Digital and Economic Affairs, and the federal states Tyrol, Salzburg and Vienna. TA was supported by the Austrian Science Fund (FWF): FP33070-B.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Ethics approval The study protocol was approved by the institutional ethics commission with an amendment to AN2017-0016 369/4.21, and written informed consent was obtained from all patients.
Provenance and peer review Not commissioned; internally peer reviewed.