Summary
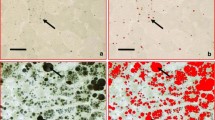
Regional distribution of glycoconjugates in normal and neoplastic colonic mucosa was studied by means of eight lectins:Dolichos biflorus (DBA),Glycine max (SBA),Triticum vulgare (WGA),Arachis hypogaea (PNA),Griffonia simplicifolia-I (GS-I),Canavalia ensiformis (Con A),Limax flavus (LFA), andUlex europaeus-I (UEA-I). The lectin binding patterns were examined in 40 normal colonic mucosa (NM) (12 proximal (P) and 28 distal (D)), 38 carcinomas (15 P and 23 D), and 31 transitional mucosa (TM) (9 P and 22 D). Sections of NM located 5 cm and 10 cm distant from the tumour and sections from the resection margins (more than 10 cm from the tumour) of the surgical specimens were also studied in 19 cases (6 P and 13 D). In NM, regional differences between the proximal and distal colon were detected with most lectins. DBA, SBA and LFA bound mainly to the goblet cell mucin of the distal colon, while GS-I and UEA-I labelling predominated in proximal colonic mucosa. The lectin reactivity in carcinomas was: DBA 26%, SBA 63%, PNA 95%, GS-I 66%, UEA-I 76%, WGA 100%, Con A 92% and LFA 42%. No regional differences were observed in the lectin patterns of proximal and distal colonic carcinomas nor was any relationship detected between lectin reactivities and Dukes stage, size or histological type of tumours. Transitional mucosa of both the proximal and distal colon showed an increase in PNA-binding and loss of DBA and SBA. LFA and UEA-I reactivity in proximal TM was similar to that observed in proximal NM. Distal TM showed a decrease in
LFA labelling and the appearance of UEA-I reactivity in goblet cell mucin in 5 cases (23%). The reactivity of the other lectins was as with NM. The only change in normal mucosa distant from tumours was a focal increase in PNA reactivity in 4 cases. These findings suggest that carcinomas from different colonic regions have a more uniform distribution of carbohydrates than the respective NM.
Similar content being viewed by others
References
Ahnen DJ, Warren GH, Greene LJ, Singleton JW, Brown WR (1987) Search for a specific marker of mucosal dysplasia in chronic ulcerative colitis. Gastroenterology 93:1346–1355
Allen A (1978) Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull 34:28–33
Berg JW, Godwin JD (1974) The epidemiologic pathology of carcinomas of the large bowel. J Surg Oncol 6:381–400
Blenkinsopp WK, Stewart-Brown S, Blesowsky L, Kearney G, Fielding LP (1981) Histopathology reporting in large bowel cancer. J Clin Pathol 34:509–513
Boland CR, Montgomery CK, Kim YS (1982) Alterations in human colonic mucin occuring with cellular differentiation and malignant transformation. Proc Natl Acad Sci USA 79:2051–2055
Bresalier BS, Boland CR, Kim YS (1985) Regional differences in normal and cancer-associated glycoconjugates of the human colon. J Natl Cancer Inst 75:249–260
Campo E, Condom E, Palacin A, Quesada E, Cardesa A (1988) Lectin binding patterns in normal and neoplastic colonic mucosa. A study ofDolichos biflorus agglutinin, Peanut agglutinin and Wheat germ agglutinin. Dis Colon Rectum 31:892–899
Clamp JR (1980) Gastrointestinal mucus. In: Wright R (ed) Recent advances in gastrointestinal pathology. Saunders Co, London, pp 47–58
Clarke AE, Hoggart RM (1982) The use of lectins in the study of glycoproteins. In: Marchalonis JJ, Warr GW (eds). Antibody as a tool. John Wiley and Sons, Chichester, pp 347–401
Compton C, Wyatt R, Konugres A, Ehrenthal D, Durda P (1987) Immunohistochemical studies of blood group substance H in colorectal tumors using a monoclonal antibody. Cancer 59:118–127
Cooper HS (1982) Peanut lectin binding sites in large bowel carcinomas. Lab Invest 47:383–390
Cooper HS, Haesler WE Jr (1978) Blood group substances as tumor antigens in the distal colon. Am J Clin Pathol 69:594–598
D'Gorman TA, LaMont JT (1978) Glycoprotein synthesis and secretion in human colon cancers and normal colonic mucosa. Cancer Res 38:2784–2789
Damjanov I (1987) Biology of disease. Lectin cytochemistry and histochemistry. Lab Invest 57:5–20
Dennis JW, Laferte S (1987) Tumor cell carbohydrate and the metastatic phenotype. Cancer Metastasis Rev 5:185–204
Filipe MI, Branfoot AC (1976) Mucin histochemistry of the colon. Curr Top Pathol 63:143–178
Freeman HJ, Kim Y, Kim YS (1978) Glycoprotein metabolism in normal proximal and distal rat colon and changes associated with 1,2-dimethylhydrazine-induced colonic neoplasia. Cancer Res 38:3385–3390
Goldstein IJ, Hughes RC, Monsigny M, Osawa T, Sharon N (1980) What should be called a lectin? Nature 285:66
Haenszel W, Correa P (1971) Cancer of the colon and rectum and adenomatous polyps. A review of epidemiologic findings. Cancer 28:14–24
Hakomori S (1981) Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Ann Rev Biochem 50:733–764
Hoskins LC, Zamcheck N (1963) Studies on gastric mucins in health and disease. Ann NY Acad Sci 106:767–774
Jacobs LR, Huber PW (1985) Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel disease. J Clin Invest 75:112–118
Kellokumpu I, Karhi K, Andersson LC (1986) Lectin-binding sites in normal, hyperplastic, adenomatous and carcinomatous human colorectal mucosa. Acta Pathol Microbiol Immunol Scand Sect A 94:271–280
Kim YS, Isaacs R (1975) Glycoprotein metabolism in inflammatory and neoplastic diseases of the human colon. Cancer Res 35:2092–2097
Klein PJ, Osmers R, Vierbuchen M, Ortman M, Kanio J, Unlenbruck G (1981) The importance of lectin binding sites and carcinoembryonic antigen with regard to normal, hyperplastic, adenomatous and carcinomatous colonic mucosa. Rec Res Cancer Res 79:1–9
Leathem AJC, Atkins NJ (1983) Lectin binding to paraffin sections. In: Bullock GR, Petrusz P (eds) Techniques in immunocitochemistry. Academic Press, London, pp 39–67
Lee YS (1987) Lectin reactivity in human large bowel. Pathology 19:397–401
Miller RL, Collawn JF, Fish WW (1982) Purification and macromolecular properties of a sialic acid-specific lectin from the slugLimax flavus. J Biol Chem 257:7574–7580
Montero C, Segura DI (1980) Retrospective histochemical study of mucosubstances in adenocarcinomas of the gastro-intestinal tract. Histopathology 4:281–291
Morson BC, Sobin LH (1976) Histological typing of intestinal tumours. International Histological Classification of Tumours No 15. WHO, Geneva
Norusis MJ (1985) SPSSx Advanced statistics guide. McGraw-Hill, New York
Örntoft TF, Mors NPO, Eriksen G, Jacobsen NO, Poulsen HS (1985) Comparative immunoperoxidase demonstration of T-antigens in human colorectal carcinomas and morphologically abnormal mucosa. Cancer Res 45:447–452
Paulson JC, Prieels JP, Glasgow LR, Hill RL (1978) Sialyland fucosyltransferases in the biosynthesis of asparaginyl-linked oligosaccharide in glycoproteins: Mutually exclusive glycosylation byβ-galactoside α2–6 sialytransferase and N-acetylglucosamide α1–3 fucosyltransferase. J Biol Chem 253:5617–5624
Pihl E, Peura A, Johnson WR, McDermott FT, Hughes ESR (1985) T-antigen expression by peanut agglutinin staining relates to mucosal dysplasia in ulcerative colitis. Dis Colon Rectum 28:11–17
Reid PE, Culling CFA, Dunn WL (1973) Saponification-induced increase in the periodic acid Schiff reaction in the gastrointestinal tract. Mechanism and distribution of the reactive substance. J Histochem Cytochem 21:473–482
Reid PE, Culling CFA, Dunn WL, Ramey CW, Clay MG (1984) Chemical and histochemical studies of normal and diseased human gastrointestinal tract. I. A comparison between histologically normal colon, colonic tumours, ulcerative colitis and diverticular disease of the colon. Histochem J 16:235–251
Rogers CM, Cooke KB, Filipe MI (1978) Sialic acids of human large bowel mucosa: O-acylated variants in normal and malignant status. Gut 19:587–592
Shamsuddin AKM, Weis L, Phelps PC (1981) Colon epithelium. IV. Human colon carcinogenesis. Changes in human colon mucosa adjacent to and remote from carcinomas of the colon. J Natl Cancer Inst 66:413–419
Shulte BA, Spicer SS, Miller RL (1984) Histochemical localization of sialoglycoconjugates with a sialic acid-specific lectin from the slugLimax flavus. Histochem J 16:1125–1132
Trump BF, Phelps PC, Shamsuddin AM (1984) Cellular pathobiology of human large intestine. In: Wolman SR, Mastromarino AJ (eds) Progress in cancer research and therapy. Raven Press, New York, pp 23–49
Urbanski SJ, Levine ME, Borgs P, Bruce WR, Krepinsky JJF (1983) Colonic mucus in human fetal development. Oncodevelop Biol Med 4:363–370
Welch JP, Donaldson GA (1979) The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg 189:496–502
Wiley EL, Mendelsohn G, Eggleston J (1981) Distribution of carcinoembryonic antigens and blood group substances in adenocarcinoma of the colon. Lab Invest 44:507–513
Wood CB, Dawson PM, Habib NA (1985) The sialomucin content of colonic resection margins. Dis Colon Rectum 28:260–261
Yonezawa S, Nakamura T, Tanaka S, Sato E (1982) Glycoconjugate withUlex europaeus agglutinin-I-binding sites in normal mucosa, adenoma, and carcinoma of the human large bowel. J Natl Cancer Inst 69:777–785
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Calderó, J., Campo, E., Ascaso, C. et al. Regional distribution of glycoconjugates in normal, transitional and neoplastic human colonic mucosa. Vichows Archiv A Pathol Anat 415, 347–356 (1989). https://doi.org/10.1007/BF00718637
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00718637