Abstract
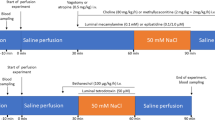
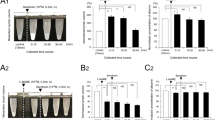
In the present study, we addressed the problem whether sympathoadrenal mechanisms could influence the paracellular permeation of macromolecules from the lumen to the lamina propria of the small intestine. Experiments were conducted with rats that were anesthetized with ether for 10–20 min, during which time laparotomy was performed and six consecutive loops (each of 5 cm length) of the jejunum were prepared. A 3% solution of the azo dye, Evans blue (EB; MW 960.83) in phosphate-buffered saline,was instilled into each loop at a volume of 0.3 ml, this compound serving as a marker for tight junctional permeability. Thereafter, the abdomen was closed and the rats were allowed to wake up, but were killed after 60 min. The loops were dissected, opened, and rinsed with acetylcysteine in order to remove the adherent mucus layer. Each loop was weighed and incubated with formamide for 24 hr to elute the amount of EB absorbed, which was quantitated spectrophotometrically. In the control situation, the uptake was homogenous along the loops. β-Adrenoceptor-blocking, or-stimulating agents could influence the uptake considerably. The data obtained could indicate that noradrenergic nerves, via an activation ofβ 2-adrenoceptors, may cause an increase of tight junction permeability for macromolecules, but circulatory mechanisms also must be taken into account in order to explain the observed effects.
Similar content being viewed by others
References
Powell DW: Barrier function of epithelia. Am J Physiol 241:G275-G288, 1981.
Hollander D: The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol 27:721–726, 1992
Madara JL Tight junction dynamics: Is paracellular transport regulated? Cell 53:497–498, 1988
Madara JL Pathobiology of the intestinal epithelial barrier. Am J Pathol 137:1273–1281, 1990
Pappenheimer JR, Dahl CE, Karnovsky ML, Maggio JE: Intestinal absorption and excretion of octapeptides composed of D amino acids. Proc Natl Acad Sci USA 91:1942–1945, 1994
Soergel KH: Showdown at the tight junction. Gastroenterology 105:1247–1250, 1993
Madara JL: Sodium-glucose cotransport and epithelial permeability. Gastroenterology 107:319, 1994
Sadowski DC, Meddings, JB: Luminal nutrients alter tightjunction permeability in the rat jejunum: Anin vivo perfusion model. Can J Physiol Pharmacol 71:835–839, 1993
Peeters M, Ghoos Y, Maes B, Hiele M, Geboes K, Vantrappen G, Rutgeerts P: Increased permeability of macroscopically normal small bowel in Crohn's disease. Dig Dis Sci 39:2170–2176, 1994
Lange S, Delbro DS, Jennische E: Evans Blue permeation of intestinal mucosa in the rat. Scand J Gastroenterol 29:38–46, 1994
Keast JR: Mucosal innervation and control of water and ion transport in the intestine. Rev Physiol Bioche, Pharmacol 109:1–59, 1987
Maxwell RA: Guenethidine after twenty years: A pharmacologist's perspective. Br J Clin Pharmacol 13:35–44, 1982
Lange S: A rat model for anin vivo assay of enterotoxic diarrhoea. FEMS Microbiol Lett 15:239–242, 1982
Siegel S, Castellan JR: Nonparametric Statistics for the Behavioral Sciences. Singapore, McGraw-Hill, 1988, 399 pp
Furness JB, Costa M: The Enteric Nervous System. Edinburgh. Churchill Livingstone, 1987, 290 pp
Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M: Effects of diethyl ether, halothane, ketamie and urethane on sympathetic activity in the rat. Eur J Pharmacol 134:15–24, 1987
Cook HJ, Reddix RA:Neural regulation of intestinal electrolyte transport.In Physiology of the Intestinal Tract, 3rd ed. LR Johnson (ed). New York, Raven Press, 1994, pp 2083–2132
Kewenter J: The vagal control of the jejunal and ileal motility and blood flow. Acta Physiol Scand 65(suppl 251):1–68, 1965
Rimele TJ, O'Dorisio MS, Gaginella TS: Evidence for muscarinic receptors on rat colonic epithelial cells: binding of [3H]-quinuclidinyl benzilate. J Pharmacol Exp Ther 218:426–434, 1981
Brunsson I, Eklund S, Jodal M, Lundgren O, Sjövall H: The effect of and sympathetic nerve activation on net water absorption in the cat's small intestine. Acta Physiol Scand 106:61–68, 1979
Thomas EM, Templeton D: Sites of noradrenergic innervation of the jejunal villus. Gen Pharmacol 13:67–69, 1982
Thomas EM, Munday KA: Sympathetic innervation of the jejunal villus. Acta Histochem 72:85–89, 1983
Altura BM, Zweifach BW: Evidence for beta receptors in the rat mesenteric microvasculature. J Pharmacol Exp Ther. 150:23–25, 1965
Henrich H, Singbartl G, Biester J: Adrenergic-induced vascular adjustments—initial and escape reactions. I. Influence of β-adrenergic blocking agents on the intestinal circulation of the rat (in vivo). Pfluegers Arch 346:1–12, 1974
Blankesteijn WM, Thien T: Effect ofN G-monomethyl-L-arginine on the β-adrenoceptor-mediated relaxation of rat mesenteric resistance arteries. Life Sci 52:PL135-PL139, 1993
Patel P, Bose D, Greenway C: Effects of prazosin and phenoxybenzamine on α- and β-receptor-mediated responses in intestinal resistance and capacitance vessels. J Cardiovasc Pharmacol 3:1050–1059, 1981
Persson CGA: The action of β-receptors on microvascular endothelium or: Is airways plasma exudation inhibited by β-agonists? Life Sci 52:2111–2121, 1993
Mahe L, Chapelain B, Gargouil YM, Neliat G: Characterization of β-adrenoceptor subtypes and indications for two cell populations in isolated bovine mesenteric lymphatic vessels. Eur J Pharmacol 199:19–25, 1991
Yeo CJ, Couse NF, Antiohos C, Zinner MJ: The effect of norepinephrine on intestinal transport and perfusion pressure in the isolated perfused rabbit ileum. J Surg Res 44:617–624, 1988
Neutra MR, O'Malley LJ, Specian RD: Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol 242:G380-G387, 1982
Nakaki T, Nakadate T, Yamamoto S, Kato R: Alpha2-adrenergic receptor in intestinal epithelial cells. Idenficacation by [3H]yohimbine and failure to inhibit cyclic AMP accumulation. Mol Pharmacol 23:228–234, 1983
Cooke HJ: Neurobiology of the intestinal mucosa. Gastroenterology 90:1057–1081, 1986
Gutnisky A, Borda ES, Gimeno AL: Are β-adrenoceptors involved in the intestinal absorption and tissue distribution of vasoclilatation iron in the rat? Eur J Pharmacol 100:369–372, 1984
Groot JA, Bakker R, Dekker K, Oosterhuis WP: Modulation of transepithelial ion permeability by neurohumoral agents; comparison of fish and rat intestine.In Ion Gradient-Coupled Transport. INSERM Symposium No. 26. F Alvarado, CH van Os (eds). Amsterdam, Elsevier Science Publishers, 1986, pp 411–414
Bakker R, Dekker K, De Jonge HR, Groot JA: VIP, serotonin and epinephrine modulate the ion selectivity of tight junctions of goldfish intestine. Am J Physiol 264:R362-R368, 1993
Kawabe H, Takai N: Autonomic regulation of tight junctional permeability in the rat submandibular gland. J Osaka Dent Univ 24:121–134, 1990
Mazariegos MR, Tice LW, Hand AR: Alteration of tight junctional permeability in the rat parotid gland after isoproterenol stimulation. J Cell Biol 98:1865–1877, 1984
Morgan KG, Angel F, Schmalz PF, Szurszewski JH: Intracellular electrical activity of muscularis mucosae of the dog stomach. Am J Physiol 249:G256-G263, 1985
van der Vliet A, Rademaker B, Bast A: A beta adrenoceptor with atypical characteristics is involved in the relaxation of the rat small intestine. J Pharmacol Exp Ther 255:218–226, 1990
Nakamaru M, Jackson EK, Inagami T: β-adrenoceptor-mediated release of angiotensin II from mesenteric arteries. Am J Physiol 250:H144-H148, 1986
Kuemmerle JF, Smith EH, Borum EH, Kellum JM: β-Adrenoceptors on duodenal mucosal cells mediate venous serotonin release. J Surg Res 44:740–744, 1988
Author information
Authors and Affiliations
Additional information
The study was supported by the Swedish Farmers Foundation for Agriecltural Research (project no. 922903). Ismelin was a kind gift from Ciba-Geigy AB, Västra Frölunda, Sweden.
Rights and permissions
About this article
Cite this article
Lange, S., Delbro, D.S. Adrenoceptor-mediated modulation of evans blue dye permeation of rat small intestine. Digest Dis Sci 40, 2623–2629 (1995). https://doi.org/10.1007/BF02220451
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02220451