Abstract
Aim/hypothesis
We determined the effect of exercise training on insulin sensitivity and muscle lipids (triglyceride [TGm] and long-chain fatty acyl CoA [LCACoA] concentration) in patients with Type 2 diabetes.
Methods
Seven patients with Type 2 diabetes and six healthy control subjects who were matched for age, BMI, % body fat and VO2peak participated in a 3 days per week training program for 8 weeks. Insulin sensitivity was determined pre- and post-training during a 120 min euglycaemic-hyperinsulinaemic clamp and muscle biopsies were obtained before and after each clamp. Oxidative enzyme activities [citrate synthase (CS), β-hydroxy-acyl-CoA (β-HAD)] and TGm were determined from basal muscle samples pre- and post training, while total LCACoA content was measured in samples obtained before and after insulin-stimulation, pre- and post training.
Results
The training-induced increase in VO2peak (~20%, p<0.01) was similar in both groups. Compared with control subjects, insulin sensitivity was lower in the diabetic patients before and after training (~60%; p<0.05), but was increased to the same extent in both groups with training (~30%; p<0.01). TGm was increased in patients with Type 2 diabetes (170%; p<0.05) before, but was normalized to levels observed in control subjects after training. Basal LCACoA content was similar between groups and was unaltered by training. Insulin-stimulation had no detectable effect on LCACoA content. CS and β-HAD activity were increased to the same extent in both groups in response to training (p<0.001).
Conclusion/interpretation
We conclude that the enhanced insulin sensitivity observed after short-term exercise training was associated with a marked decrease in TGm content in patients with Type 2 diabetes. However, despite the normalization of TGm to levels observed in healthy individuals, insulin resistance was not completely reversed in the diabetic patients.
Similar content being viewed by others
Type 2 diabetes is characterized by skeletal muscle insulin resistance and impaired glucose metabolism. In addition to disturbances in glucose homeostasis, individuals with Type 2 diabetes have an impaired lipid metabolism, reflected by increased circulating free fatty acids [1], reduced rates of whole body fat oxidation [2] and excessive deposition of lipids in various tissues including skeletal muscle [3]. With regard to muscle lipid status, there is evidence in humans that increased trigacylglycerol (TGm) concentration [3, 4, 5, 6, 7] and increased long-chain fatty acyl CoA (LCACoA) content are negatively associated with whole body insulin action [8]. Considering the relationship between skeletal muscle lipid accretion and insulin resistance, it is of particular interest to determine whether interventions such as exercise training can reduce muscle lipid content and improve whole body insulin action.
In this regard, several recent studies have reported that short-term (4–10 weeks) aerobic exercise training programs were effective in improving whole body aerobic capacity and insulin sensitivity in both young, healthy subjects [9] and overweight and obese men [10], independent of any changes in TGm content. Indeed, exercise training has been shown to increase TGm content in muscle from healthy individuals [11], suggesting that the presence of triacyglycerol in the myocyte is not always associated with insulin insensitivity. In addition, it has been reported that the capacity to oxidize TGm and/or VLDL-derived fatty acids is increased both at rest and during exercise following a 3-month low intensity (40% of VO2peak) training program, and that increased rates of TGm oxidation could be beneficial in the prevention/treatment of Type 2 diabetes [12]. However, to date the effects of exercise training on insulin sensitivity and muscle lipid status in Type 2 diabetic patients has not been determined. Accordingly, the purpose of this study was to investigate the effect of a short-term (8-week) program of aerobic exercise training on TGm and LCACoA concentration in Type 2 diabetic individuals. We hypothesized that exercise training would result in a reduction in muscle lipid stores and that such changes would parallel improvements in insulin sensitivity.
Subjects and methods
Subjects
We studied seven male patients with Type 2 diabetes with a mean time since diagnosis of disease of 2.9±0.5 years (range 1 to 5 years) and a control group consisting of six healthy male subjects matched for age, BMI, % body fat and VO2peak (Table 1). Normal glucose tolerance was confirmed in control subjects by an OGTT (75 g glucose). The patients were being treated with diet (n=1) or oral hypoglycaemic agents (n=1 sulphonylureas, n=2 metformim, n=3 combination of sulphylureas and metformim). Other than diabetes, none of these subjects had any medical problems, all were non-smokers and apart from diabetic patients, none were taking medications known to alter carbohydrate metabolism. All subjects had maintained a constant body mass (BM) during the 6 months preceeding the experiment and were not participating in any form of regular physical activity. As the principal aim of the investigation was to examine the effects of exercise training per se, subjects were studied under free-living conditions and were instructed not to make any dietary changes throughout the study. This has been common practice in a number of other investigations [9, 10]. The experimental protocol was approved by the Human Ethics Committee of RMIT University. The purpose, nature and potential risks of the study were explained to all subjects and written informed consent was obtained prior to participation.
Euglycaemic-hyperinsulinaemic clamp
Whole body insulin mediated glucose uptake was determined at 08:00 h following a 12–14 h overnight fast, 1 week before and 36–48 h after completion of the training program. Subjects were instructed to refrain from any form of vigorous physical activity for 72 h prior to the determination of pre-training insulin sensitivity. An anticubital vein was cannulated for infusion of glucose and insulin, and a hand vein was cannulated retrogradely and was heated for sampling of arterialized blood. After collection of baseline blood samples (5 ml), a percutaneous biopsy of the v. lateralis muscle was obtained. Briefly, local anesthaesia [1% lignocaine (lidocaine)] was administered to the skin, subcutaneous tissue and fascia of the v. lateralis and an incision was made. At this time a second site on the same leg (5 cm distal) was prepared for the post-clamp biopsy. Then, a resting muscle biopsy was obtained using a 6 mm Bergström needle modified to include suction. Muscle biopsy specimens (100–150 mg) were immediately frozen in liquid nitrogen and stored at −80°C until subsequent analysis. After resting for 5 min, a primed (9 mU·kg−1) continuous infusion of insulin (Actrapid, Novo Nordisk, Sydney, Australia) was commenced at a rate of 40 mU·m−2·min−1, and blood glucose concentration was measured at 5-min intervals throughout the clamp. A variable rate infusion of 20% glucose was used to maintain euglycaemia (5 mmol/l) for the duration of the clamp (120 min). The blood glucose concentration in the diabetic subjects was allowed to decrease during the insulin infusion to 5 mmol/l and then maintained at this concentration for the remainder of the clamp. Samples (2 ml) for subsequent determination of plasma insulin concentration were obtained every 10 min, spun in a centrifuge and stored at −80°C. Whole body glucose uptake was calculated from the glucose infusion rate (mg·kg FFM−1·min−1) required to maintain a blood glucose concentration of 5 mmol/l throughout the last 30 min of the clamp. A second muscle biopsy was obtained 120 min after the initiation of the insulin infusion. To prevent a decrease in plasma potassium concentration during the clamp, 30 mmol KCl (Slow-K; Novartis, Sydney, Australia) was administered orally. A 120-min clamp was chosen as a similar protocol has previously been used to determine insulin sensitivity in patients with Type 2 diabetes [13]. However, there is the possibility that a clamp of this duration might not completely suppress hepatic glucose production in insulin-resistant patients. Accordingly, the glucose infusion rate for the Type 2 diabetic and control subjects might not have been at a steady state for the same length of time during the clamp.
Determination of peak oxygen uptake (VO2peak)
All subjects carried out a progressive, incremental cycling test to volitional fatigue on an electronically-braked ergometer (Lode, Groningen, The Netherlands) for determination of VO2peak prior to, at the midpoint, and at the completion of the training program (described below).
Assessment of body composition
Whole body dual energy X-ray absorptiometry (DEXA; Lunar DPX, Lunar Radiation, Madison, Wis., USA), 1 week before and after the training program, was used to measure total and regional tissue composition (body fat, lean tissue mass). Prior to each scan, the DEXA was calibrated with known phantoms.
Exercise training program
The exercise-training program consisted of 8 weeks of ergometer cycling undertaken 3 days per week under supervision in the laboratory. Over the initial 4 weeks, subjects exercised a minimum of twice a week at 70% of pre-training VO2peak, so that by the end of week 4 they were cycling continuously for 60 min. On the third day of training, and after a brief (10 min) warm-up, subjects carried out short exercise bouts (~3 min) of intense (~80 of VO2peak) cycling separated by 2 min of active recovery (~50% of VO2peak), for a total exercise time of about 50 min. The number of exercise bouts during interval training was progressively increased so that by the completion of week 4 subjects were able to do eight work bouts. After week 4 of exercise training, VO2peak was reassessed and the training program adjusted, so that for the remaining 4 weeks subjects cycled for about 60 min at 70% of their ‘new’ VO2peak twice a week, while the high intensity interval training session was undertaken at 85% of VO2peak with the same work-to-rest interval. At the completion of the 8-week training program, subjects carried out a third and final VO2peak test. Whole body rates of carbohydrate and fat oxidation were determined from indirect calorimetry during a 20-min ride at 65% of pre-training VO2peak during the first and last week of the program. During every training session, ratings of perceived exertion and heart rate were monitored (Polar Electro, Oy, Finland). During the training program, heart rate averaged 135±2 beats·min−1 for control subjects and 127±14 beats·min−1 for Type 2 diabetic patients, while the average ratings of perceived exertion averaged over all training sessions was 13±1 for control subjects and 12±1 for the patients with Type 2 diabetes. Subjects were allowed free access to water during the training sessions.
Blood biochemistry
Blood glucose concentration was measured using an automated analyzer (Yellow Springs Instruments 2300 Stat Plus Glucose and L-Lactate Analyzer, Yellow Springs, Ohio, USA). Plasma insulin concentration was determined by radioimmunoassay using a commercially available kit (LINCO Human Insulin Specific RIA Kit, St. Charles, Mo., USA). HbA1C was determined by specific ion-exchange chromatography (Sigma Diagnostics, NSW, Australia ). Plasma non-esterified free fatty acid (NEFA) concentration was determined using an enzymatic colourimetric method (NEFA C test kit, Wako, Richmond, Va., USA). Plasma cholesterol and triglycerides were analyzed using an automated analyzer (Cholestech LDX, Cholestech, Hayward, Calif., USA).
Intramuscular triglyceride concentration
Approximately 30 mg of muscle was freeze-dried under vacuum for 24 h. The sample (~10 mg) was viewed under a microscope (×6.3) at room temperature for dissection and removal of all traces of adipose tissue, connective tissue and blood contaminants. This procedure yielded approximately 7–8 mg of dry weight dissected muscle from which a direct measure of TGm content was determined, as previously described [5]. Total lipids were extracted according to a known method of [14] from the dried and dissected skeletal muscle in 4 ml of chloroform:methanol (2:1) and left to rotate at room temperature overnight. We added 0.6% sodium chloride and centrifugation (2000 rpm for 10 min) resulted in a separation of the aqueous and organic phases. The organic phase containing the TG was transferred to a glass vial and air dried. The isolated lipids were re-suspended in 250 µl of ethanol and the triglyceride concentration determined spectrophotometrically at 490 nm using an enzymatic colourimetric test kit (Triglycerides GPO-PAP, Boehringer Mannheim, Sydney, Australia). The within-assay coefficient of variation for this assay in our laboratory is 8%.
Tissue LCACoA measurement
The total amount of LCACoA in muscle biopsy samples was measured using an assay adapted from another study [15]. Tissue was homogenized in 10% tirchloroacetic acid. The acid insoluble pellet (which includes proteins, some lipids and LCACoAs) was washed with ether and water to remove unwanted lipids and water-soluble contaminants. The pellet was re-suspended in 10 mmol/l DTT and the pH raised to 11.5 with KOH. The sample was incubated at 55°C for 10 min to hydrolyse the ester bond between fatty acid and CoA. After hydrolysis the sample was neutralized with 1 mol/l HCl, centrifuged at 13 000 rpm and 250 µl of the supernatant used for the determination of CoA. The supernatant was added to a reaction buffer containing 50 mmol/l KH2PO4, 100 µmol/l α-ketoglutarate, 50 µmol/l NAD+. After a basal reading 20 mU of α-ketoglutarate dehydrogenase was added and the reaction rate was followed to completion using a flourimeter (340 nm excitation, 460 nm emission). The concentration of CoA in the sample was calculated with reference to a standard curve of known concentrations of CoA. The within-assay coefficient of variation for this assay in our laboratory is 10%.
Citrate synthase and β-hydroxy-acyl-CoA dehydrogenase (β-HAD) activity
Muscle (5–10 mg) was homogenized in 1:50 dilution (w/v) of a 175 mmol/l potassium buffer solution and citrate synthase activity was assayed spectrophotometrically at 25°C as previously described [16]. β-HAD activity was assayed spectrophotometrically at 25°C measuring the disappearance of NADH using the same homogenate as for citrate synthase [17].
Statistical analysis
Data are presented as means±SEM. Differences were determined using a two-way (group×training) ANOVA for all measures except the effect of insulin-stimulation and training on LCACoA content. For this measure a three-way (group×clamp×training) ANOVA was used. Significant differences were located using a Newman Kuels post hoc-test. A p value of less than 0.05 was considered statistically significant.
Results
Subject characteristics
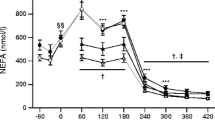
BM, BMI and percentage body fat were similar between the two experimental groups (Table 1) and did not change after the training programme. The clinical characteristics of all subjects are shown in Table 2. There was a main effect for fasting blood glucose concentration, being higher in patients with Type 2 diabetes compared with control subjects (p<0.01). Training resulted in a reduction in fasting plasma insulin (p<0.05) and plasma NEFA levels (p<0.05). HbA1C was increased in the diabetic patients (p<0.01). Plasma triglycerides, cholesterol, HDL, LDL and VLDL were similar between the two experimental groups (Table 2) and did not change after the training program.
Effect of exercise training on aerobic capacity and rates of whole body substrate oxidation
The 8 weeks of endurance exercise training increased VO2peak by about 20% (p<0.001) and the magnitude of this increase was similar in both groups. During submaximal cycling at 65% of VO2peak, whole body rates of fat oxidation were lower in Type 2 diabetic patients compared with control subjects (0.24±0.02 vs 0.58±0.10 g/min; p<0.05). In the patients with Type 2 diabetes, the exercise training program resulted in an increase in the rate of fat oxidation (0.24±0.02 vs 0.32±0.01 g/min; p<0.05) and a concomitant reduction in the rate of carbohydrate oxidation (2.32±0.18 vs 1.77±0.11 g/min; p<0.05) during a 20 min standardized ride at 65% of pre-training VO2peak (~55% of post-training VO2peak). Unfortunately, due to technical difficulties we were only able to determine rates of substrate oxidation in two control subjects following training. In those two individuals the rates of carbohydrate oxidation were 2.36±0.09 g/min, while whole body rates of fat oxidation were 0.46±0.02 g/min.
Whole body insulin-stimulated glucose uptake
There were no significant differences in blood glucose (Fig. 1A) or plasma insulin (Fig. 1B) concentration during the final 30 min of the clamp procedure. Whole body insulin-stimulated glucose uptake was impaired in patients with Type 2 diabetes [60% reduction compared to control subjects (p<0.05)] before the exercise training intervention (Fig. 2). Despite a similar relative increase in insulin sensitivity in both groups after exercise training (~30%), whole body insulin-stimulated glucose uptake remained markedly impaired (60%) in the Type 2 diabetic subjects.
Whole body insulin-stimulated glucose infusion rate (GIR) averaged over 10 min intervals as measured throughout the euglycaemic-hyperinsulinaemic clamp before (pre) and after (post) an 8-week exercise training intervention (a) and steady state GIR determined during the final 30 min of the euglycaemic-hyperinsulinaemic clamp (b). Values are means±SEM. *Significant main effect of group, p<0.001. †Significant main effect of training, p<0.01
Skeletal muscle lipids
TGm content was about 170% higher in muscle from Type 2 diabetic individuals compared with control subjects [89.0±23.2 vs 33.3±4.3 mmol/kg dry weight (dw); p<0.05; Fig. 3]. Exercise training resulted in a reduction (47%) in TGm content in Type 2 diabetic subjects (89.0±23.2 vs 47.5±11.4 mmol/kg d.w.; p<0.05), but not in control subjects. Basal skeletal muscle LCACoA content was similar between groups (Table 3). LCACoA content was not altered by the exercise training program. Insulin-stimulation had no detectable effect on total LCACoA content [~12 nmol/g wet weight (ww); Table 3], although there was a tendency for LCACoA content to be suppressed following insulin-stimulation in control subjects post-training (11.0±0.8 vs 9.6±0.8 nmol/g w.w.; p=0.07).
Intramuscular triglyceride (TGm) content in resting biopsy samples obtained from the v. lateralis before (pre) and after (post) an 8 week exercise training Intervention. Values are means±SEM. *-Significantly different to pretaining, p<0.05. †-Significantly different to subjects with Type 2 diabetes at same time point, p<0.05
Skeletal muscle oxidative capacity
The activity of citrate synthase increased in response to training (main effect p<0.001) in skeletal muscle from both Type 2 diabetic (73%) and control subjects (85%; Table 4). Training resulted in an (main effect p<0.001) increase in β-HAD activity (Type 2 diabetic patients: 62%; control subjects: 48%; Table 4).
Discussion
Evidence is accumulating to suggest that together with hyperglycaemia, Type 2 diabetes is associated with profound alterations in lipid metabolism. In particular, accretion of lipid in skeletal muscle seems to play a crucial role in the aetiology of insulin resistance: a number of independent research groups using a variety of different measurement techniques have reported negative correlations between TGm and insulin action [3, 4, 5, 6, 7]. Here we report that exercise training in Type 2 diabetic individuals resulted in a “normalization” of TGm content, but did not completely reverse insulin resistance in these patients.
It is well known that endurance training improves insulin action in healthy individuals [9], with an increase in insulin sensitivity being observed after only 7 days of training [18, 19, 20]. The mechanisms by which exercise training improves insulin action in healthy skeletal muscle have been attributed to increases in insulin-stimulated PI3-kinase activity [18], increased expression of GLUT4 [21], hexokinase [22] and glycogen synthase [22]. Exercise training has also been shown to increase insulin sensitivity in patients with Type 2 diabetes [23]. In this study, exercise training resulted in a similar increase in insulin sensitivity in both Type 2 diabetic and healthy control subjects matched for age, BMI, percentage of body fat and VO2peak. However, despite the similar relative increase in insulin sensitivity, whole body insulin-stimulated glucose uptake remained markedly impaired in the Type 2 diabetic subjects.
The effects of exercise training on TGm content in healthy subjects are equivocal, with some studies reporting an increase [11, 24, 25], decrease [26] or no change [9, 10, 27] after short-term (6–12 weeks) endurance exercise. In our investigation, exercise training had little effect on TGm concentrations in control subjects, but resulted in a substantial overall decrease in patients with Type 2 diabetes. Indeed, patients with the highest basal (pre-training) levels of TGm seemed to have the largest decrease after the exercise training intervention. However, despite TGm content being “normalized” to that of control subjects following the training program, insulin resistance (i.e., the glucose infusion rate during the clamp) was not fully reversed in the diabetic patients. Indeed, despite relatively large changes in lipid status following exercise training, the resting blood glucose concentration in patients with Type 2 diabetes was virtually unaltered. As glucose toxicity seems to reduce whole body insulin-stimulated glucose disposal in diabetic patients [28], this could contribute to the persistent insulin resistance observed in Type 2 diabetic subjects in this study following the exercise training intervention. Precisely how sustained hyperglycaemia produces insulin resistance is unknown. However, taken collectively, our findings strongly suggest that TGm content is merely a reflection of the metabolic events that lead to a decreased insulin sensitivity rather than a direct causal factor of this state. Thus, muscle triacylglycerol content might merely act as a surrogate measure for other lipid-derived moieties that could have a more mechanistic and direct link with the pathophysiology of insulin resistance, such as the fatty acid composition of membrane phospholipids [9], diacylglycerol (DAG) [29] or the metabolically active LCACoA [30].
Investigations of the measurement of LCACoA in humans are scarce, but a recent study reported increased LCACoA content in skeletal muscle from overweight/obese and extremely obese individuals compared with normal weight subjects [31]. Furthermore, total LCACoA content has been reported to be a better correlate of insulin action than measurements of TGm [8]. Hence, a second novel finding of the present investigation was that despite markedly different levels of insulin sensitivity and increased TGm content in patients with Type 2 diabetes, total LCACoA at rest was similar between Type 2 diabetic and control subjects. Furthermore, 8 weeks of exercise training did not result in any detectable difference in resting muscle LCACoA content despite a reduction in TGm and enhanced whole body insulin sensitivity. These findings are in agreement with those of another study [10] that reported that the improvement in insulin sensitivity following moderate intensity exercise was independent of changes in muscle LCACoA levels in overweight and obese men. Although speculative, it is possible that the endurance training intervention undertaken by subjects in the present investigation resulted in changes in the individual LCACoA species (i.e., a reduction in the saturated species). In support of such a contention, exercise training has been shown to increase the degree of unsaturation of skeletal muscle membranes [9, 32, 33], suggesting that regular exercise modifies the fatty acid profile of skeletal muscle in favour of enhanced insulin sensitivity.
In the present study we were unable to detect any reduction in LCACoA content in muscle from Type 2 diabetic patients following acute insulin-stimulation either before or after training. This is in agreement with our previous findings that LCACoA content was not suppressed during a hyperinsulinaemic-euglycaemic clamp in a group of individuals with varying degrees of insulin sensitivity [34]. However, in healthy control subjects there was a tendency for insulin stimulation to suppress LCACoA content, with this decrease approaching statistical significance post-training (p=0.07). Although tentative, these data are suggestive that a suppression of LCACoA content could be associated with enhanced insulin sensitivity in muscle from healthy individuals. A common feature of both diet- and/or exercise-induced reversal of insulin resistance is a decrease in the muscle LCACoA levels during a glucose clamp [35]. Accordingly, it might have been predicted that insulin stimulation would have a greater potential to suppress LCACoA content in the Type 2 diabetic patients rather than the control subjects, as insulin sensitivity in these patients was markedly increased after the training intervention. We have no explanation why insulin stimulation would suppress LCACoA content in only healthy compared to insulin-resistant muscle. However, it should be remembered that although exercise training markedly improved insulin sensitivity in the Type 2 diabetic subjects, whole body insulin-stimulated glucose uptake was still markedly impaired compared to the control subjects.
It is well known that endurance training elicits an increase in the oxidative capacity of skeletal muscle from healthy, insulin-sensitive subjects [25, 36, 37]. Thus, regular exercise training might be therapeutic in overcoming derangements in lipid metabolism observed in Type 2 diabetes. Indeed, we observed a training-induced increase in muscle oxidative capacity that was of the same order of magnitude in both Type 2 diabetic and control subjects. This improved “metabolic fitness” was associated with an enhanced capacity for fatty acid utilization in the diabetic patients, reflected by an increase in whole body rates of fat oxidation during submaximal exercise conducted at the same absolute pre-training workload. Previous studies have also shown that a program of endurance training similar in intensity and duration to that used in the present investigation, results in an increase in whole body fat oxidation [27, 38]. Furthermore, in healthy subjects a short-term, low-intensity exercise training program has recently been shown to increase the capacity to oxidize TGm and/or VLDL-derived fatty acids, both at rest and during submaximal exercise [12]. It does not seem unreasonable, therefore, to suggest that part of the training-induced increase in rates of whole body fat oxidation during exercise in the Type 2 diabetic patients in the current study would have resulted in increased rates of TGm oxidation (and turnover) during the actual exercise-training sessions, and that this, in part, could be responsible for the reduction in resting TGm levels.
Finally, both Type 2 diabetic patients and the matched control subjects in this investigation were overweight/obese. However, the difference in the average body mass of participants in the two groups was not statistically significant. Notwithstanding this fact, the Type 2 diabetic subjects were about 15 kg heavier than the control subjects, and such a difference in body mass could have an influence on some of our findings. For example, it is likely that if the subjects were leaner, there could have been greater improvements in whole-body insulin-stimulated glucose utilization. While weight loss alone can reduce insulin resistance, physical activity (i.e., exercise training) and weight loss are generally recommended as a combined intervention as there is uncertainty regarding their respective effects on improvements in insulin resistance [10].
In conclusion, we observed that enhanced insulin action following 8 weeks of exercise training was associated with an increase in whole body rates of fat oxidation and a marked decrease in muscle TGm content in patients with Type 2 diabetes. However, despite the “normalization” of TGm concentration to values observed in healthy control subjects, insulin resistance was not completely reversed in the diabetic patients. These findings provide compelling evidence that TGm per se does not confer insulin resistance, but possibly acts as a surrogate for other lipid-derived metabolites, such as diacylglycerol, that have a more mechanistic link with the development of insulin resistance.
Abbreviations
- β-HAD:
-
β-hydroxy-acyl-CoA
- CS:
-
citrate synthase
- DAG:
-
diacylglycerol
- DEXA:
-
dual energy X-ray absorptiometry
- LCACoA:
-
long-chain fatty acyl CoA
- PI3-kinase:
-
phosphatidylinositol 3-kinase
- TGm :
-
muscle triacylglycerol
References
Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD (1988) Measurement of plasma glucose, free fatty acid, lactate and insulin for 24 h in patients with NIDDM. Diabetes 37:1020–1024
Kelley DE, Goodpaster B, Wing RR, Simoneau J-A (1999) Skeletal muscle fatty acid metabolism in assocation with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277:E1130–E1141
Pan DA, lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH (1997) Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46:983–988
Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsle U, McKeigue PM, Bell JD (1999) Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia 42:932–935
Kriketos AD, Furler SM, Gan SK, Poynten AM, Chisholm DJ, Campbell LV (2003) Multiple indexes of lipid availability are independently related to whole body insulin action in healthy humans. J Clin Endocrinol Metab 88:793–798
Krssak M, Peterson KF, Dresner A, DiPietro L, Vogel SM, Rothman DL, Shulman GI, Roden M (1999) Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a1H NMR spectroscopy study. Diabetologia 42:113–116
Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L (1999) Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans. A1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of Type 2 diabetic parents. Diabetes 48:1600–1606
Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, Cooney GJ (2000) Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab 279:E554–E560
Helge JW, Dela F (2003) Effect of training on muscle triacylglycerol and structural lipids. A relation to insulin sensitivity? Diabetes 52:1881–1887
Gan SK, Kriketos AD, Ellis BA, Thompson CH, Kraegen EW, Chisholm DJ (2003) Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care 26:1706–1713
Schrauwen-Hinderling VB, Schrauwen P, Hesselink MKC, van Engelshoven JMA, Nicolay K, Saris WHM, Kessels AGH, Kooi ME (2003) The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab 88:1610–1616
Schrauwen P, van Aggel-Leijssen DPC, Hul G, Wagenmakers AJM, Vidal H, Saris WHM, Baak MA van (2002) The effect of a 3-month low-intensity endurance training program on fat oxidation and acetyl-CoA carboxylase-2 expression. Diabetes 51:2220–2226
Ryder JW, Yang J, Galuska D, Rincon J, Bjornholm M, Krook A, Lund S, Pedersen O, Wallberg-Henriksson H, Zierath JR, Holman GD (2000) Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes 49:647–54
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB (1998) Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem 273:16146–16154
Srere PA (1969) Citrate synthase. Methods Enzymol 13:3–11
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York
Houmard JA, Shaw CD, Hickey MS, Tanner CJ (1999) Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in human skeletal muscle. Am J Physiol Endocrinol Metab 277:E1055–E1060
Cox JH, Cortright RN, Dohm GL, Houmard JA (1999) Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol 86:2019–2025
Youngren JF, Keen S, Kulp JL, Tanner CJ, Houmard JA, Goldfine ID (2001) Enhanced muscle insulin receptor autophosphorylation with short-term aerobic exercise training. Am J Physiol Endocrinol Metab 280:E528–E553
Houmard JA, Shinebarger MH, Dolan PL, Leggett-Frazier N, Bruner RK, McCammon MR, Israel RG, Dohm GL (1993) Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol Endocrinol Metab 264:E896–E901
Hjeltnes N, Galuska D, Björnholm M, Aksnes A-K, Lannem A, Zierath JR, Wallberg-Henriksson H (1998) Exercise-induced overexpression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanisms for improved glucose homeostasis. FASEB J 12:1701–1712
Dela F, Larsen JJ, Mikines KJ, Ploug T, Peterson LN, Galbo H (1995) Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes 44:1010–1020
Kiens B, Éssen-Gustavsson B, Christensen NJ, Saltin B (1993) Skeletal muscle substrate utilization during submaximal exercise in man: Effect of endurance training. J Physiol 469:459–478
Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM (1996) Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol Endocrinol Metab 270:E265–E272
Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA (1999) Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol Endocrinol Metab 276:E106–E117
Hurley BF, Nemeth PM, Martin WH III, Hagberg JM, Dalsky GP, Holloszy JO (1986) Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol 60:562–567
Yki-Järvinen H, Helve E, Koivisto V (1987) Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes 36:892–896
Itani SI, Ruderman NB, Schmieder F, Boden G (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51:2005–2011
McGarry JD (2002) Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18
Hulver MW, Berggren JR, Cortright RL, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA (2003) Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284:E741–E747
Andersson A, Sjödin A, Hedman A, Olsson R, Vessby B (2000) Fatty acid profile of skeletal muscle phospholipids in trained and untrained young men. Am J Physiol Endocrinol Metab 279:E744–E751
Thomas TR, Londeree BR, Gerhardt KO, Gehrke CW (1977) Fatty acid profile and cholesterol in skeletal muscle of trained and untrained men. J Appl Physiol 43:709–713
Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA (2003) Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 88:
Cooney GJ, Thompson Al, Furler SM, Ye J, Kraegen EW (2002) Muscle long-chain acyl CoA esters and insulin resistance. Ann NY Acad Sci 967:196–207
Gollnick PD, Armstrong RB, Saltin B, Saubert CW, 4th., Sembrowich WL, Shepherd RE (1973) Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34:107–111
Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO (1992) Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 72:1780–1786
Aggel-Leijssen DPC van, Saris WHM, Wagenmakers AJM, Senden JM, Baak MA van (2002) Effect of exercise training at different intensities on fat metabolism of obese men. J Appl Physiol 92:1300–1309
Acknowledgements
We gratefully acknowledge the research volunteers for their participation in this difficult study. We also wish to thank M.J. Anderson, A.L. Carey, S.A. Clark and K. Greenway for technical assistance, and the scientific input of Dr R.M. Bektash and Associate Professor M.A. Febbraio. This study was supported by Masterfoods, Australia/New Zealand, a Mars, incorporated company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruce, C.R., Kriketos, A.D., Cooney, G.J. et al. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with Type 2 diabetes. Diabetologia 47, 23–30 (2004). https://doi.org/10.1007/s00125-003-1265-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1265-7