Abstract
Each human intestine harbours not only hundreds of trillions of bacteria but also bacteriophage particles, viruses, fungi and archaea, which constitute a complex and dynamic ecosystem referred to as the gut microbiota. An increasing number of data obtained during the last 10 years have indicated changes in gut bacterial composition or function in type 2 diabetic patients. Analysis of this ‘dysbiosis’ enables the detection of alterations in specific bacteria, clusters of bacteria or bacterial functions associated with the occurrence or evolution of type 2 diabetes; these bacteria are predominantly involved in the control of inflammation and energy homeostasis. Our review focuses on two key questions: does gut dysbiosis truly play a role in the occurrence of type 2 diabetes, and will recent discoveries linking the gut microbiota to host health be helpful for the development of novel therapeutic approaches for type 2 diabetes? Here we review how pharmacological, surgical and nutritional interventions for type 2 diabetic patients may impact the gut microbiota. Experimental studies in animals are identifying which bacterial metabolites and components act on host immune homeostasis and glucose metabolism, primarily by targeting intestinal cells involved in endocrine and gut barrier functions. We discuss novel approaches (e.g. probiotics, prebiotics and faecal transfer) and the need for research and adequate intervention studies to evaluate the feasibility and relevance of these new therapies for the management of type 2 diabetes.
Similar content being viewed by others
Introduction
The onset of type 2 diabetes is clearly associated with both host genetics and environmental factors (e.g. diet, physical activity). Emerging evidence indicates that the risk of developing type 2 diabetes may involve a particular environmental factor, specifically, the collection of microorganisms that inhabit our intestine. Each human intestine harbours not only hundreds of trillions of bacteria, but also bacteriophage particles, viruses, fungi and archaea, which constitute a complex and dynamic ecosystem with which we live in symbiosis throughout our lifetime [1]. Given that host genetics is thought to contribute to the profile of the gut microbiome, all living conditions, including dietary habits, exposure to xenobiotics (such as drugs, toxicants and additives) or stresses (such as surgery and infections) will modulate the gut microbiota, occasionally for a limited period of time due to the resilience of this ecosystem [2]. This review starts with a description of the human studies relating the changes in the gut microbiota to glycaemia in type 2 diabetic patients.
Dysbiosis related to type 2 diabetes and hyperglycaemia
Several clinical trials are ongoing to obtain more precise and more reliable information about the changes in the composition and function of the gut microbiota that may be specifically associated with hyperglycaemia and type 2 diabetes, independently of other contributing factors (e.g. body weight) (for a recent review, see [3]).
Metagenomic data have revealed that patients with type 2 diabetes exhibit a moderate degree of gut microbial dysbiosis compared with patients with inflammatory bowel disease [4]. The proportions of the phylum Firmicutes and the class Clostridia are significantly reduced, whereas the class of the gram-negative Betaproteobacteria is highly enriched in the faeces of type 2 diabetic patients compared with non-diabetic individuals, and the proportion of Betaproteobacteria is positively correlated with plasma glucose levels [5].
Interestingly, the microbiome of type 2 diabetic patients are characterised by the depletion of several butyrate-producing bacteria, including Clostridium species, Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia intestinalis and Roseburia inulinivorans [4, 6, 7], and an enrichment of opportunistic pathogens [4]. Bacteria increased in the gut of type 2 diabetic patients also include the sulphate-reducing bacteria Desulfovibrio, as well as Lactobacillus gasseri, Lactobacillus reuteri and Lactobacillus plantarum [6, 7]. Curiously, the treatment of Japanese type 2 diabetic patients with α-glucosidase inhibitors has been shown to increase Lactobacillus spp. [7]. In accordance with these findings, an increasing number of observational studies have reported changes in the gut microbiota associated with type 2 diabetes, but the outcomes are not always concordant. Zhang et al found a decreased abundance of Akkermansia muciniphila, a mucus-colonising bacterium that plays a role in gut barrier function, in diabetic and glucose-intolerant patients [8]; this observation has been reported in several studies of obese individuals. Data on a Chinese population indicated the opposite effect, specifically, an increase in A. muciniphila in type 2 diabetic patients [4]. Thus, it appears that genetic background and/or medication can influence the gut microbiota, which might explain discrepancies between studies.
Many articles have reported a correlation between changes in the gut microbiota and markers of type 2 diabetes. Lactobacillus species correlate positively with fasting glucose and HbA1c levels whereas Clostridium species correlate negatively with fasting glucose, HbA1c and insulin levels [6]. A recent study suggests that a higher blood glucose concentration may be predicted by a reduction in the proportion of anaerobes, particularly Bacteroides [9].
Importantly, different features of metabolic disorders, including markers of glucose metabolism disorders (i.e. insulinaemia and HOMA-IR), but not BMI or body weight, are significantly associated with the gene count of the microbiome, suggesting that individuals with a low gene count are characterised by metabolic disturbances known to increase the risk of diabetes [10].
The functions of the microbiome are also affected in type 2 diabetic patients, such as an increase in membrane transport of sugars or branched amino acids, the activity of enzymes involved in xenobiotic or carbohydrate metabolism, or sulphate reduction [4, 6]. In contrast, functions involved in cell motility, butyrate synthesis and cofactor and vitamin metabolism are decreased in type 2 diabetic patients [4, 6]. Importantly, markers related to oxidative stress resistance are also enriched in type 2 diabetic patients, suggesting a type 2 diabetes-associated increase in defence mechanisms in the gut microbiota [4, 6].
Important questions remain unanswered regarding the long-term persistence of the changes specifically associated with diabetes and the cause–effect relationship of dysbiosis with the occurrence or progression of type 2 diabetes in humans. Clearly, because the alterations in glucose metabolism can be transmitted by gut microbiota transfer in germ-free mice [11], some gut microbial populations/functions may play an active role in the pathogenesis of glucose metabolism disorders. For evident ethical reasons, the ‘transfer’ of the diabetic phenotype via the gut microbiota has never been tested in humans.
Bacterial components and metabolites prone to interact with glucose homeostasis: an overview of the molecular mechanisms underlying microbe–host interactions in the context of diabetes
A chronic low-grade inflammation in type 2 diabetes appears to be a driver of metabolic alterations linked to obesity. The inflammation in the different tissues contributes to insulin resistance. The triggers of the inflammatory response include endoplasmic reticulum stress, inflammasome activation and Toll-like receptors (TLRs). The involvement of TLRs implicates a response to bacterial elements present in the gut microbiota [12, 13].
Bacterial components involved in diabetes
Gut microbes are able to communicate with the host via specific cell membranes or related molecules that may activate pattern recognition receptors (PRRs). These PRRs are involved in the recognition of molecular patterns (known as pathogen-associated molecular patterns or PAMPs) that are specific to bacteria and other microorganisms. The most studied PRRs are the TLRs. It is understood that the stimulation of TLR-4 by bacterial lipopolysaccharides (LPS) results in an inflammatory response, cytokine production and chemokine-mediated recruitment of acute inflammatory cells [14]. In 2007, our laboratory first discovered that the gut microbiota also contributes to the onset of insulin resistance and type 2 diabetes via mechanisms associated with an increase in plasma LPS, defined as metabolic endotoxaemia [15]. In experimental obesity and type 2 diabetes, metabolic endotoxaemia is associated with an altered composition of the gut microbiota and with increased intestinal permeability [15–17]. Several human studies also reported an increase in LPS or LPS-binding protein levels in association with type 2 diabetes [18]. Taken together, these data highlight a strong relationship between the gut microbiota, inflammation and metabolic perturbations, including hyperglycaemia. More recently, we discovered that specifically inactivating a protein of the innate immune system that is involved in the signalling of most TLRs (i.e. deleting the protein myeloid differentiation primary response gene 88 [MyD88]) in intestinal cells induces body weight loss and improves type 2 diabetes associated with obesity in mice fed a high-fat diet (HFD). Importantly, this phenomenon is mediated by gut microbiota-dependent mechanisms, and these data clearly suggest that intestinal cell walls play a crucial role in the systemic metabolic response to bacterial elements [19]. The efficacy of the gut barrier is controlled by numerous pathways and cell types, including mucus-producing goblet cells, tight junction proteins, the endocannabinoid system and immune responses [20]. In addition, other bacterial components, such as peptidoglycans, which bind nucleotide-binding oligomerisation domain-containing protein 2 (NOD2) receptors, are likely to play a protective role in the control of insulin resistance and obesity. Indeed, experimental data have recently shown that inhibition of peptidoglycan signalling in Nod2 −/− mice fed an HFD provokes dysbiosis and promotes bacterial adherence in the mucosae and bacterial accumulation in the liver, thereby contributing to systemic inflammation, insulin resistance and adiposity [21]. Similarly, TLR5-deficient mice, which lose their response to bacterial flagellin in the intestinal mucosa, show mild loss of glycaemic control, which is likely to be driven by insulin resistance and partially compensated for by increased insulin production—conditions typically observed in humans with the metabolic syndrome [11]. In humans, a nonsense polymorphism (R392X) in TLR5 appears to protect against obesity but, as consistent with findings in animals, predisposes individuals to type 2 diabetes [22].
Bacterial metabolites and glucose homeostasis
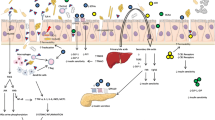
Metabolites produced by gut microbes may also be related to the development, or the control, of insulin resistance and type 2 diabetes. Most of the data illustrated in Fig. 1 have been obtained using mouse models of diabetes and obesity. As explained below, several metabolites can modulate the endocrine function of the gut, potentially affecting glucose homeostasis.
Metabolites produced by gut microbes may be related to the development or the control of insulin resistance and type 2 diabetes. The figure presents several pathways by which microbial metabolites can influence various physiological processes (such as gut barrier function, appetite, insulin secretion and response and intestinal gluconeogenesis) and thereby affect glucose homeostasis. For more details, please refer to the main text. Most of the findings illustrated in the figure have been obtained using mouse models of diabetes and obesity. The figure was produced using Servier MedicalArt (www.servier.com). 1°BA, primary bile acids; 2°BA, secondary bile acids
Short-chain fatty acids (SCFAs; e.g. butyrate, propionate and acetate) are among the most widely investigated metabolites produced by the gut microbiota that interfere with host metabolism. These molecules are produced by the microbial fermentation of specific oligo- or polysaccharides (i.e. non-digestible carbohydrates) via distinct metabolic pathways [23]. The effect of SCFAs on insulin sensitivity and energy metabolism is now widely accepted, although various physiological pathways have been suggested. Indeed, SCFAs are able to modify the levels of several gut peptides involved in glucose metabolism, gut barrier function and energy homeostasis [24–26]. For example, butyrate and propionate were shown to suppress weight gain in mice with HFD-induced obesity (DIO), and acetate was shown to reduce food intake in healthy mice [27, 28]. The majority of the pathways underlying these effects remain unknown. Several studies have suggested that the effects of SCFAs are mediated by the members of a recently identified G protein-coupled receptor family that includes G protein-coupled receptors 43 and 41 (GPR43 and GPR41, respectively) (for a review, see [29]). The binding of SCFAs to GPR43 and GPR41 increases the plasma levels of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), leading to improved glucose homeostasis and reduced appetite (for a review, see [30]). Interesting studies in animals have shown that butyrate activates the expression of genes involved in intestinal gluconeogenesis via a cAMP-dependent mechanism, whereas propionate, already known as a substrate for gluconeogenesis, promotes intestinal gluconeogenic gene expression via a gut–brain neural circuit involving GPR41. The subsequent release of glucose into the portal vein contributes to the regulation of glycaemia and insulin sensitivity [31].
Recent data have indicated that the production of indole, a metabolite produced by gut bacteria from tryptophan, may also contribute to the secretion of GLP-1 by intestinal enteroendocrine cells [32, 33]. Chimerel et al discovered that indole inhibits voltage-gated K+ channels, thereby changing the action potential properties of L cells and leading to enhanced Ca2+ entry, which acutely triggers GLP-1 secretion [34]. More importantly, it has been found that over a longer period of stimulation indole acts as an inhibitor of mitochondrial metabolism, resulting in a reduction in the intracellular ATP concentration, which induces the opening of ATP-sensitive K+ (KATP) channels, thereby hyperpolarising the plasma membrane and slowing GLP-1 release [34]. Interestingly, we recently demonstrated that among alcoholic individuals, those with higher gut permeability, higher metabolic endotoxaemia and low-grade inflammation exhibit a lower abundance of indole and 3-methyl indole [35]. Taken together, the discovery that indole may trigger GLP-1 secretion and the finding that gut barrier function is reinforced by indole, lead us to suggest that GLP-2 is involved in the control of gut barrier [36] and that its co-secretion with GLP-1 by L cells may be controlled by indoles.
Over the last 10 years, studies have demonstrated that not only are bile acids important in the digestion of dietary lipids, but they also act as signalling molecules in the context of energy, glucose and lipid metabolism [37]. A recent study has reported that pretreatment of DIO mice with antibiotics (vancomycin and bacitracin), which reduces the levels of the major bacterial phyla (Bacteroidetes and Firmicutes) in the gut and changes the production of bacterial metabolites, improves glucose intolerance and insulin resistance The authors proposed GLP-1 as a mediator of these effects and noted an increase in primary conjugated bile acid (taurocholic acid) levels as a potential key driver of GLP-1 secretion and a key regulator of host glucose homeostasis [38]. TGR5, a G protein-coupled receptor primarily localised to intestinal enteroendocrine cells, is primarily activated by secondary bile acids produced by the gut microbiota (lithocholic and deoxycholic acids). Activation of this receptor has been associated with improved liver function and glucose tolerance in obese mice by regulating intestinal GLP-1 production [37, 39, 40] (for a review, see [41]). Interestingly H2S, which can be produced by bacteria expressing sulphate-reducing enzymes, may counteract TGR5 activation and exert an inhibitory effect on GLP-1 and PYY release [42]. Moreover, studies conducted in mice have demonstrated that the gut microbiota regulate the expression of fibroblast growth factor 15 (for which the orthologous protein in humans is fibroblast growth factor 19 [FGF19]) in the gut by activating the farnesoid X receptor—these hormones are responsible for transmitting bile acid-induced signals in targeted tissues to regulate weight gain and insulin resistance [40, 43, 44]. Joyce et al have shown that promoting the activity of bile salt hydrolase (BSH)—an enzyme distributed across the major bacterial divisions and archaea that catalyses the deconjugation of bile acids to produce secondary bile acids—in the gut microbiota may directly control body weight, blood cholesterol levels, hepatic lipid levels and fat mass gain [45]. Interestingly, a recent intervention study involving the administration of a BSH-active L. reuteri strain to healthy volunteers led to an increase in total plasma (conjugated and unconjugated) bile acid levels that correlated with the serum FGF19 levels [46]. The impact of changing the availability and the profile of bile acids on host glucose homeostasis remains to be clearly established in humans, but these metabolites appear to function as important mediators of host metabolism.
Thus, although the influence of the gut microbiota on energy metabolism is multifactorial, different targets involving immunity and/or specific metabolites have been emphasised in recent studies, clearly demonstrating the rationale for searching for novel therapeutic targets based on compounds derived from or produced by bacteria.
Potential contribution of the gut microbiota to the pharmacological or surgical treatment of type 2 diabetes
The discovery of the gut microbiota as a metabolic partner in the management of type 2 diabetes also led to the publication of studies investigating whether gut microbes play a role in the benefits of type 2 diabetes therapies.
Metformin is the most widely used glucose-lowering drug. However, its mechanism of action remains unclear [47]. A first clue regarding the involvement of the gastrointestinal tract in the benefits of metformin came from the observation that intravenous administration of metformin was unable to reduce glycaemia [48]. A second clue came from the finding that the improvement in glucose tolerance induced by metformin was abrogated in mice treated with broad-spectrum antibiotics [49]. Strikingly, Shin et al reported that metformin induced a profound shift in the microbial ecosystem in favour of Akkermansia spp. and that oral administration of A. muciniphila improved glucose tolerance [49], thereby confirming the results obtained at our laboratory [50]. The authors thus suggested that a modulation of the gut microbiota (likely an increase in the Akkermansia spp. population) may contribute to the glucose-lowering effects of metformin. A few months later, Lee et al confirmed that metformin treatment induces an increase in the A. muciniphila population and demonstrated a negative correlation between glycaemia and A. muciniphila abundance. Interestingly, co-incubation of metformin and mouse stool samples led to an enrichment in A. muciniphila [51], suggesting that metformin directly interacts with the gut microbiota to foster the growth of A. muciniphila.
Acarbose, an α-glucosidase inhibitor that is almost exclusively used in Asia, is another type 2 diabetes drug with effects that could be related to the gut microbiota. In Chinese patients, the inclusion of acarbose as part of their glucose-lowering medication has been reported to increase faecal Bifidobacterium spp. and reduce LPS levels [52].
Interestingly, new therapeutic agents proposed for the treatment of type 2 diabetes (sitagliptin and exenatide) exploit the GLP-1 pathway. As mentioned earlier, GLP-1 secretion can also be stimulated by metabolites produced by the gut microbiota [25]. Reimer et al demonstrated that co-administration of sitagliptin and a viscous fermentable fibre, which is broken down into SCFA, more effectively reduced fasting glycaemia in obese Zucker rats than either treatment alone [53]. Similar results were obtained in the same model when this fibre was combined with metformin or with metformin and sitagliptin [54].
Currently, the combination of medical therapy with bariatric surgery (vertical sleeve gastrectomy [VSG] or Roux-en-Y gastric bypass [RYGB]) appears to more effectively control glycaemia than medical therapy alone in obese patients with uncontrolled diabetes [55]. In this context, studies have found that RYGB restructures the gut microbiota in humans and rats [56, 57]. Transfer of the gut microbiota of mice that underwent RYGB to non-operated germ-free mice resulted in weight loss and decreased fat mass but no change in fasting glycaemia, providing the first evidence that changes in the gut microbiota contribute to the metabolic improvements conferred by RYGB [56]. As explained above, bile acids might link the gut microbiota to the host. Their levels are modified after bariatric surgery, and VSG does not improve hyperglycaemia in mice carrying a targeted genetic deletion of the farnesoid X receptor, implicating bile acids as bacterial modulators of host homeostasis in this context [58]. Bile acids are without doubt very interesting mediators. However, the differences in bile acid and cholesterol metabolism between mice and humans make it difficult to translate the data from the animal models to the human situation.
Novel therapeutic approaches of type 2 diabetes based on the understanding of gut microbiota–host interactions
Aside from the classical treatments, the recently recognised implication of gut microbes in the physiopathology of type 2 diabetes opens a novel area of research for developing new strategies to tackle this disease using gut microbes.
Microbiota transfer
An original study recently investigated this approach using an infusion of faecal microbiota from lean donors to recipients with the metabolic syndrome [59]. The transfer of a microbiota sample from healthy patients was able to increase the levels of butyrate-producing bacteria and insulin sensitivity in insulin-resistant recipients [59], thus suggesting that the isolation of the microbiota from faecal content might be developed as a therapeutic strategy to increase insulin sensitivity in humans. However, this type of experiment assessing the role of the gut microbiota in the control of diabetes in humans is currently a proof-of-concept rather than a potential therapy. Additional studies are needed to confirm the lack of harmful effects linked to the transfer of faecal microorganisms, most of which are unidentified and uncharacterised at present.
Probiotic approach
More specific approaches may also be considered for type 2 diabetic patients. Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit to the host (i.e. humans) [60]. To date, the major probiotic strains that have shown beneficial effects on glucose metabolism in humans belong to the Lactobacillus genus (i.e. L. plantarum 299v, Lactobacillus acidophilus NCFM and L. gasseri SBT2055) [61–63]. These observations may appear to be discordant, as some Lactobacillus species have been shown to be increased in type 2 diabetic patients, as previously discussed. However, the increase in Lactobacillus species in type 2 diabetes has never been demonstrated to have a direct impact on the disease. Moreover, the effects obtained using probiotics are probably strain-specific; thus, different strains of the same species may exert distinct effects. Importantly, it could be interesting to investigate other ‘beneficial’ microorganisms that are decreased in diabetic patients.
Among the bacteria that could potentially be used for the treatment of type 2 diabetes, A. muciniphila appears to be of particular interest. By administering A. muciniphila MucT (ATTC BAA-835) in a diet-induced mouse model of type 2 diabetes, we demonstrated the direct beneficial effects of this bacterium on glucose metabolism [50]. First, A. muciniphila is able to counteract fasting hyperglycaemia in diet-induced mouse model of type 2 diabetes by preventing the increase in G6pc (glucose-6-phosphatase) mRNA expression [50]. This suggests that A. muciniphila thwarts the deleterious increase in gluconeogenesis in diabetic mice. Moreover, administration of live A. muciniphila alleviates glucose intolerance in HFD-induced diabetic mice [49, 50]. However, additional studies are needed to establish whether A. muciniphila can be used as a probiotic for patients with type 2 diabetes, and of these, intervention studies in humans are of utmost importance. Finally, A. muciniphila is probably not the sole bacterium that could be beneficial for the treatment of these patients; other bacteria, such as F. prausnitzii, which plays an important role in the maintenance of the gut barrier and in the control of inflammation, could also be interesting to investigate (for a review, see [64]).
Non-bacterial ‘colonisers’ of the gut of potential interest
In addition to the classical probiotic bacteria, several other types of living organism might contribute to the therapeutic arsenal for treating hyperglycaemia in the future. Here, we consider the current knowledge on fungi, archaea and helminths regarding their relationship with host glycaemia.
Our understanding of the contribution of the mycobiota (fungal community) to health and disease remains in its infancy [65]. Our laboratory recently provided the first evidence supporting the hypothesis that fungi can influence host metabolism. The yeast Saccharomyces boulardii changed the gut microbiota and reduced certain features of the metabolic syndrome in genetically obese and diabetic mice. However, this yeast did not change fasting glycaemia in these mice [66]. Improving our understanding of the mycobiota and its relationship with the host might lead in the future to the development of new therapies for the metabolic syndrome.
The predominant archaeon member in the human gut is Methanobrevibacter smithii. How this methanogenic archaeon collaborates with saccharolytic bacteria such as Bacteroides thetaiotaomicron to metabolise complex carbohydrates was elegantly established almost 10 years ago [67]. This symbiotic association increases adiposity when inoculated into germ-free mice [67]. In humans, methanogenic archaea are increased in obese vs lean individuals [68], and intestinal methane production in obese individuals is associated with a higher BMI [69]. However, this association cannot be generalised to all archaea [70], and their relationship with glycaemia has not been reported.
Helminths are known to induce T helper type 2-oriented immunity in association with eosinophilia. For this reason, Nippostrongylus brasiliensis has been used in a mouse model of DIO to maintain eosinophil homeostasis in adipose tissue, and this intervention led to reduced adipose macrophage counts and fasting glucose levels [71]. In accordance with these results, metabonomic investigation of mice infected with Schistosoma mansoni suggested a stimulation of glycolysis, which might also contribute to the glucose-lowering effect associated with helminth infection [72]. Moreover, as helminths influence the gut microbiota (e.g. increased lactobacilli) [73, 74], we cannot exclude an indirect effect of helminths on host metabolism via modulation of the gut microbiota. Voluntary infection with helminths might not constitute an appropriate therapeutic approach to reducing blood glucose levels. However, unravelling the biological mechanisms underlying the beneficial effects of helminths on glucose metabolism (such as the induction of eosinophilia or the stimulation of the growth of lactobacilli) should reveal new therapeutic targets and would help to identify how the gut ecosystem plays a role in the control of host metabolism.
A place for nutrition in the management of glycaemia-related dysbiosis
Inulin-type fructans
Nutrition plays an important role in the management of diabetes. Indeed, some nutrients are able to decrease the postprandial glucose response. Cereals, legumes, fruits and spices are four important food groups that contain active ingredients (such as dietary fibre and polyphenols) that are able to reduce glycaemia and insulin responses in humans [75]. The glucose-lowering effect of fibre intake may depend on the fibre type, amount and/or source. Dietary inulin-type fructans (ITF), which are present in various fruits and vegetables, are fermentable carbohydrates that display prebiotic properties, as their metabolisation by gut microorganisms modulates the composition and/or activity of the gut microbiota, thus conferring a beneficial physiological effect on the host [76]. ITF increase the number of endocrine L cells in the jejunum and colon of rodents and promote the production and release of the active forms of GLP-1, thereby decreasing glycaemia [77–81]. A systematic review conducted to evaluate the effectiveness of dietary ITF on serum glucose in humans revealed that four out of 13 eligible randomised controlled trials published from 1984 to 2009 reported a decrease in serum glucose concentrations [82]. Interestingly, in healthy volunteers, 2 weeks of treatment with ITF (16 g per day) increased the postprandial release of gut peptides (specifically GLP-1 and gastric inhibitory peptide), modified eating behaviour (increased satiety and decreased energy intake) and decreased postprandial glycaemia [83]. One study performed on a limited number of patients at risk for cardiovascular disease did not support the effect of ITF on insulin sensitivity [84]. Short-chain-enriched inulin (10 g/day) caused a significant decrease in the levels of fasting plasma glucose, HbA1c and inflammatory markers (IL-6, TNF-α and LPS) compared with maltodextrin in a trial of 52 overweight type 2 diabetes women over a period of 8 weeks [85]. In a study of the correlations between glycaemic control by ITF in obese women and gut bacteria, changes in Clostridium cluster IV group (which was increased by ITF) were negatively correlated with fasting glycaemia, insulinaemia and HOMA-IR [86]. In contrast, changes in Propionibacterium spp., Bacteroides intestinalis and Bacteroides vulgatus, all three of which were significantly decreased by prebiotic treatment, were positively correlated with the changes in glucose homeostasis. Serum LPS levels were negatively correlated with several bacterial phyla and species, specifically Firmicutes, Actinobacteria, Bifidobacterium and F. prausnitzii, all of which were promoted by ITF. The promotion of Bifidobacterium by ITF is logical since these bacteria express β-fructosidase, but the other changes, such as the interesting increase in F. prausnitzii, remain unexplained.
Arabinoxylans
Other non-digestible carbohydrates are gradually fermented throughout the colon, and these might have beneficial health effects by acting as substrates for certain microbes. Arabinoxylans (AX), the most abundant non-digestible carbohydrates in wheat, are predominantly present in bran and aleurone fractions [87, 88]. AX are selectively degraded in the colon by intestinal bacteria expressing xylanases and arabinofuranosidases and represent a new class of prebiotics [89–91]. Table 1 summarises the findings of studies on AX and AXOS (short-chain AX produced via enzymatic processing) in animal models and in humans. In our studies, AX and AXOS supplementation induced caecal and colon enlargement, increased Bifidobacterium spp., Bacteroides/Prevotella spp. and Roseburia spp. and improved insulin resistance in a diet-induced mouse model of type 2 diabetes [92, 93]. Importantly, correlation analysis revealed that the Roseburia spp. levels are inversely correlated with HOMA-IR and inflammatory markers. AXOS increased the level of GLP-1 and counteracted the HFD-induced increase in HOMA-IR. In addition, AXOS reduced HFD-induced metabolic endotoxaemia [92]. Most human intervention studies, including those of type 2 diabetic patients, assessing the effects of wheat-derived AX(OS) on glucose metabolism demonstrated a decrease in glycaemia (Table 1). Additional studies are needed to determine whether the effect of AX(OS) on gut microbiota is linked to the improvement in glucose homeostasis.
Polyphenols
Some phenolic compounds abundant in fruit, vegetables, chocolate, nuts and beverages (tea, coffee, wine and soy milk) may be poorly absorbed in the upper part of the gut and are fermented by bacteria in the colon. Our laboratory has demonstrated that supplementation with pomegranate peel extract, which is rich in ellagitannins and anthocyanins, modulates the gut microbiota in favour of bifidobacteria [94]. Although this effect was accompanied by the reduced expression of key inflammatory factors, it did not significantly modify glycaemia or glucose tolerance. Of note, several recent studies have highlighted the importance of gut microbiota modulation in the metabolic effects of polyphenols on glucose homeostasis. One such polyphenol is resveratrol, a natural phytoalexin present in red grapes, peanuts, and berries that displays antioxidant and anti-inflammatory properties. In one study, resveratrol increased GLP-1 production via a mechanism that was dependent on the alteration of the intestinal microbiota and required the GLP-1 receptor to mediate its antidiabetic effect on DIO mice. In particular, it was shown that Parabacteroides johnsonii, Alistipes putredinis and Bacteroides vulgatus, the levels of which were increased by HFD treatment, disappeared 5 weeks after resveratrol supplementation [95]. In another study, mice fed an HFD supplemented with 4% green tea powder for 8 weeks had a significantly increased insulin response compared with control mice [96]. In addition, fasting plasma glucose, insulin and HOMA-IR levels were lower in mice fed the green tea supplement for 11 or 22 weeks. In a third study, the administration of cranberry extract, which is rich in proanthocyanidins, improved insulin sensitivity in high-fat/high-sucrose diet-fed mice. In this study, cranberry extract treatment markedly increased the proportion of Akkermansia and decreased intestinal inflammation [97]. Finally, a double-blind trial revealed that changes in the gut microbiota are associated with the glucose-lowering effects of a traditional berberine-containing Chinese herbal formula in type 2 diabetic patients [98]. Indeed, this decoction significantly increased F. prausnitzii, which was negatively correlated with fasting blood glucose, HbA1c and postprandial blood glucose levels and was positively correlated with HOMA of beta cell function.
Importantly, energy-free artificial sweeteners were extensively introduced to our diets with the intention of reducing energy intake and normalising blood glucose levels without ‘sweet-toothed’ humans having to compromise. A recent study demonstrated that the consumption of commonly used artificial sweetener formulations drives the development of glucose intolerance via the induction of compositional and functional alterations to the intestinal microbiota [99]. Whether the bacterial populations or metabolic pathways altered by the consumption of artificial sweeteners are similar to those described in individuals with or developing diabetes remains to be elucidated [99, 100].
Conclusions
Type 2 diabetes, a complex disease that is often associated with obesity, develops via the interaction between genetic and environmental factors. We believe that the gut microbiota represents an environmental factor of type 2 diabetes that was neglected in the past due to the complexity of its analysis and to the lack of an understanding of the mechanisms underlying the interactions between gut microbes and host metabolism. The current interest in the gut microbiota as a potential target for the management of non-communicable diseases such as type 2 diabetes partially relies on the novel methodologies available for analysing the composition and function of the gut microbiota, as well as on the recent discoveries of host molecular targets that are prone to ‘respond’ to bacterial metabolites/components. To those who might question the relevance of gut dysbiosis in the occurrence of type 2 diabetes, we would say that all of the data supporting a causative role of dysbiosis in type 2 diabetes have been obtained using germ-free animals into which the intestinal content of diabetic mice was transferred. As far as the development of novel therapeutic approaches is concerned, intervention studies using probiotic, prebiotic, or microbial transplantation have been successful in a very limited number of published reports. Nutritional advice is crucial in the management of diabetes. We believe that a better characterisation of the nutrients that are able to modulate the gut microbiota in favour of anti-inflammatory bacteria or bacterial metabolites is needed to provide adequate advice to patients who are at risk for type 2 diabetes development.
Abbreviations
- AX:
-
Arabinoxylans
- AXOS:
-
Arabinoxylan oligosaccharides
- BSH:
-
Bile salt hydrolase
- DIO:
-
Diet-induced obesity
- FGF19:
-
Fibroblast growth factor 19
- GLP:
-
Glucagon-like peptide
- GPR:
-
G protein-coupled receptor
- HFD:
-
High-fat diet
- ITF:
-
Inulin-type fructans
- LPS:
-
Lipopolysaccharides
- PRR:
-
Pattern recognition receptor
- PYY:
-
Peptide YY
- RYGB:
-
Roux-en-Y gastric bypass
- SCFA:
-
Short-chain fatty acid
- TLR:
-
Toll-like receptors
- TGR5:
-
Transmembrane G protein-coupled receptor 5
- VSG:
-
Vertical sleeve gastrectomy
References
Hoffmann C, Dollive S, Grunberg S et al (2013) Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One 8, e66019
Goodrich JK, Waters JL, Poole AC et al (2014) Human genetics shape the gut microbiome. Cell 159:789–799
Allin KH, Nielsen T, Pedersen O (2015) Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol 172:R167–R177
Qin J, Li Y, Cai Z et al (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60
Larsen N, Vogensen FK, van den Berg FW et al (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5, e9085
Karlsson FH, Tremaroli V, Nookaew I et al (2013) Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498:99–103
Sato J, Kanazawa A, Ikeda F et al (2014) Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 37:2343–2350
Zhang X, Shen D, Fang Z et al (2013) Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One 8, e71108
Sepp E, Kolk H, Loivukene K, Mikelsaar M (2014) Higher blood glucose level associated with body mass index and gut microbiota in elderly people. Microb Ecol Health Dis. doi:10.3402/mehd.v25.22857
Le Chatelier E, Nielsen T, Qin J et al (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546
Vijay-Kumar M, Aitken JD, Carvalho FA et al (2010) Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328:228–231
Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA (2015) Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes 6:598–612
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Beutler B (2004) Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257–263
Cani PD, Amar J, Iglesias MA, et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 1761-1772
Cani PD, Bibiloni R, Knauf C et al (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481
Cani PD, Neyrinck AM, Fava F et al (2007) Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50:2374–2383
Sun L, Yu Z, Ye X et al (2010) A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33:1925–1932
Everard A, Geurts L, Caesar R et al (2014) Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun 5:5648
Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD (2014) Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benefic Microbes 5:3–17
Denou E, Lolmede K, Garidou L et al (2015) Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med 9:259–274
Al-Daghri NM, Clerici M, Al-Attas O et al (2013) A nonsense polymorphism (R392X) in TLR5 protects from obesity but predisposes to diabetes. J Immunol 190:3716–3720
Reichardt N, Duncan SH, Young P et al (2014) Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 8:1323–1335
Reimann F, Tolhurst G, Gribble FM (2012) G-protein-coupled receptors in intestinal chemosensation. Cell Metab 15:421–431
Tolhurst G, Heffron H, Lam YS et al (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61:364–371
Plaisancie P, Dumoulin V, Chayvialle JA, Cuber JC (1995) Luminal glucagon-like peptide-1(7-36) amide-releasing factors in the isolated vascularly perfused rat colon. J Endocrinol 145:521–526
Lin HV, Frassetto A, Kowalik EJ Jr et al (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7, e35240
Frost G, Sleeth ML, Sahuri-Arisoylu M et al (2014) The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5:3611
Bindels LB, Dewulf EM, Delzenne NM (2013) GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci 34:226–232
Everard A, Cani PD (2014) Gut microbiota and GLP-1. Rev Endocr Metab Disord 15:189–196
De Vadder F, Kovatcheva-Datchary P, Goncalves D et al (2014) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96
Yokoyama MT, Carlson JR (1979) Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am J Clin Nutr 32:173–178
DeMoss RD, Moser K (1969) Tryptophanase in diverse bacterial species. J Bacteriol 98:167–171
Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F (2014) Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep 9:1202–1208
Leclercq S, Matamoros S, Cani PD et al (2014) Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 111:E4485–E4493
Cani PD, Possemiers S, Van de WT et al (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103
Thomas C, Gioiello A, Noriega L et al (2009) TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10:167–177
Hwang I, Park YJ, Kim YR et al (2015) Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J 29:2397–2411
Claus SP, Tsang TM, Wang Y et al (2008) Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol 4:219
Sayin SI, Wahlstrom A, Felin J et al (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235
Prawitt J, Caron S, Staels B (2011) Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep 11:160–166
Bala V, Rajagopal S, Kumar DP et al (2014) Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-ε pathway and modulated by endogenous H2S. Front Physiol 5:420
Li F, Jiang C, Krausz KW et al (2013) Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4:2384
Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A (2014) Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr–Fgf15 axis in mice. Cell Rep 7:12–18
Joyce SA, MacSharry J, Casey PG et al (2014) Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 111:7421–7426
Martoni CJ, Labbe A, Ganopolsky JG, Prakash S, Jones ML (2015) Changes in bile acids, FGF-19 and sterol absorption in response to bile salt hydrolase active L. reuteri NCIMB 30242. Gut Microbes 6:57–65
Rena G, Pearson ER, Sakamoto K (2013) Molecular mechanism of action of metformin: old or new insights? Diabetologia 56:1898–1906
Bonora E, Cigolini M, Bosello O et al (1984) Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects. Curr Med Res Opin 9:47–51
Shin NR, Lee JC, Lee HY et al (2014) An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63:727–735
Everard A, Belzer C, Geurts L et al (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071
Lee H, Ko G (2014) Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol 80:5935–5943
Su B, Liu H, Li J et al (2014) Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. doi:10.1111/1753-0407.12232
Reimer RA, Grover GJ, Koetzner L et al (2012) Sitagliptin reduces hyperglycemia and increases satiety hormone secretion more effectively when used with a novel polysaccharide in obese Zucker rats. J Nutr 142:1812–1820
Reimer RA, Grover GJ, Koetzner L, Gahler RJ, Lyon MR, Wood S (2014) Combining sitagliptin/metformin with a functional fiber delays diabetes progression in Zucker rats. J Endocrinol 220:361–373
Schauer PR, Kashyap SR, Wolski K et al (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366:1567–1576
Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM (2013) Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5:178ra41
Aron-Wisnewsky J, Dore J, Clement K (2012) The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol 9:590–598
Ryan KK, Tremaroli V, Clemmensen C et al (2014) FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509:183–188
Vrieze A, van Nood E, Holleman F et al (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143:913–916
Hill C, Guarner F, Reid G et al (2014) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Naruszewicz M (1998) Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis 137:437–438
Andreasen AS, Larsen N, Pedersen-Skovsgaard T et al (2010) Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 104:1831–1838
Ogawa A, Kadooka Y, Kato K, Shirouchi B, Sato M (2014) Lactobacillus gasseri SBT2055 reduces postprandial and fasting serum non-esterified fatty acid levels in Japanese hypertriacylglycerolemic subjects. Lipids Health Dis 13:36
Miquel S, Martin R, Bridonneau C et al (2014) Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes 5:146–151
Mukherjee PK, Sendid B, Hoarau G, Colombel JF, Poulain D, Ghannoum MA (2015) Mycobiota in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol 12:77–87
Everard A, Matamoros S, Geurts L, Delzenne NM, Cani PD (2014) Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio 5:e01011–e01014
Samuel BS, Gordon JI (2006) A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A 103:10011–10016
Zhang H, DiBaise JK, Zuccolo A et al (2009) Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 106:2365–2370
Basseri RJ, Basseri B, Pimentel M et al (2012) Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol (N Y) 8:22–28
Fernandes J, Wang A, Su W et al (2013) Age, dietary fiber, breath methane, and fecal short chain fatty acids are interrelated in Archaea-positive humans. J Nutr 143:1269–1275
Wu D, Molofsky AB, Liang HE et al (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332:243–247
Wang Y, Holmes E, Nicholson JK et al (2004) Metabonomic investigations in mice infected with Schistosoma mansoni: an approach for biomarker identification. Proc Natl Acad Sci U S A 101:12676–12681
Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB (2010) Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis 16:1841–1849
Reynolds LA, Smith KA, Filbey KJ et al (2014) Commensal-pathogen interactions in the intestinal tract: lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes 5:522–532
Thondre PS (2013) Food-based ingredients to modulate blood glucose. Adv Food Nutr Res 70:181–227
Bindels LB, Delzenne NM, Cani PD, Walter J (2015) Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12:303–310
Cani PD, Dewever C, Delzenne NM (2004) Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr 92:521–526
Delzenne NM, Cani PD, Daubioul C, Neyrinck AM (2005) Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr 93(Suppl 1):S157–S161
Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM (2005) Involvement of endogenous glucagon-like peptide-1(7-36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol 185:457–465
Cani PD, Neyrinck AM, Maton N, Delzenne NM (2005) Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res 13:1000–1007
Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R (2006) Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55:1484–1490
Bonsu NK, Johnson CS, McLeod KM (2011) Can dietary fructans lower serum glucose? J Diabetes 3:58–66
Cani PD, Lecourt E, Dewulf EM et al (2009) Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 90:1236–1243
Tripkovic L, Muirhead NC, Hart KH, Frost GS, Lodge JK (2014) The effects of a diet rich in inulin or wheat fibre on markers of cardiovascular disease in overweight male subjects. J Hum Nutr Diet. doi:10.1111/jhn.12251
Dehghan P, Pourghassem Gargari B, Asghari Jafar-abadi M (2014) Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition 30:418–423
Dewulf EM, Cani PD, Claus SP et al (2013) Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62:1112–1121
Neyrinck AM, Delzenne NM (2010) Potential interest of gut microbial changes induced by non-digestible carbohydrates of wheat in the management of obesity and related disorders. Curr Opin Clin Nutr Metab Care 13:722–728
Andersson AA, Andersson R, Piironen V et al (2013) Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chem 136:1243–1248
Grootaert C, Van den Abbeele P, Marzorati M et al (2009) Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol 69:231–242
Vardakou M, Palop CN, Christakopoulos P, Faulds CB, Gasson MA, Narbad A (2008) Evaluation of the prebiotic properties of wheat arabinoxylan fractions and induction of hydrolase activity in gut microflora. Int J Food Microbiol 123:166–170
Hughes SA, Shewry PR, Li L, Gibson GR, Sanz ML, Rastall RA (2007) In vitro fermentation by human fecal microflora of wheat arabinoxylans. J Agric Food Chem 55:4589–4595
Neyrinck AM, Van Hee VF, Piront N et al (2012) Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes 2, e28
Neyrinck AM, Possemiers S, Druart C et al (2011) Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 6, e20944
Neyrinck AM, Van Hee VF, Bindels LB, De BF, Cani PD, Delzenne NM (2013) Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr 109:802–809
Dao TM, Waget A, Klopp P et al (2011) Resveratrol increases glucose induced GLP-1 secretion in mice: a mechanism which contributes to the glycemic control. PLoS One 6, e20700
Axling U, Olsson C, Xu J et al (2012) Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 9:105
Anhe FF, Roy D, Pilon G et al (2014) A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64:872–883
Xu J, Lian F, Zhao L et al (2015) Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J 9:552–562
Suez J, Korem T, Zeevi D et al (2014) Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514:181–186
Feehley T, Nagler CR (2014) Health: the weighty costs of non-caloric sweeteners. Nature 514:176–177
Vogel B, Gallaher DD, Bunzel M (2012) Influence of cross-linked arabinoxylans on the postprandial blood glucose response in rats. J Agric Food Chem 60:3847–3852
Christensen KL, Hedemann MS, Laerke HN et al (2013) Concentrated arabinoxylan but not concentrated beta-glucan in wheat bread has similar effects on postprandial insulin as whole-grain rye in porto-arterial catheterized pigs. J Agric Food Chem 61:7760–7768
Hartvigsen ML, Jeppesen PB, Laerke HN, Njabe EN, Knudsen KE, Hermansen K (2013) Concentrated arabinoxylan in wheat bread has beneficial effects as rye breads on glucose and changes in gene expressions in insulin-sensitive tissues of Zucker diabetic fatty (ZDF) rats. J Agric Food Chem 61:5054–5063
Lu ZX, Gibson PR, Muir JG, Fielding M, O’Dea K (2000) Arabinoxylan fiber from a by-product of wheat flour processing behaves physiologically like a soluble, fermentable fiber in the large bowel of rats. J Nutr 130:1984–1990
Lu ZX, Walker KZ, Muir JG, O'Dea K (2004) Arabinoxylan fibre improves metabolic control in people with type II diabetes. Eur J Clin Nutr 58:621–628
Garcia AL, Otto B, Reich SC et al (2007) Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur J Clin Nutr 61:334–341
Garcia AL, Steiniger J, Reich SC et al (2006) Arabinoxylan fibre consumption improved glucose metabolism, but did not affect serum adipokines in subjects with impaired glucose tolerance. Horm Metab Res 38:761–766
Mohlig M, Koebnick C, Weickert MO et al (2005) Arabinoxylan-enriched meal increases serum ghrelin levels in healthy humans. Horm Metab Res 37:303–308
Maki KC, Gibson GR, Dickmann RS et al (2012) Digestive and physiologic effects of a wheat bran extract, arabino-xylan-oligosaccharide, in breakfast cereal. Nutrition 28:1115–1121
Hartvigsen ML, Laerke HN, Overgaard A, Holst JJ, Bach Knudsen KE, Hermansen K (2014) Postprandial effects of test meals including concentrated arabinoxylan and whole grain rye in subjects with the metabolic syndrome: a randomised study. Eur J Clin Nutr 68:567–574
Funding
NMD is a recipient of FRS-FNRS grants (CDR J.0122.15, 1.5121.12F), of grants from the Walloon Region (FOOD4GUT project, convention 1318148; CAPPLE project, convention 6605; NUTRIGUTIOR project, convention 6918), of the European Union’s Seventh Framework Program community (KBBE.2013.2.2-02 MyNewGut project) and of IWT subsidies (SBO-project). PDC, a research associate at the Fonds de la Recherche Scientifique (FRS-FNRS), Belgium, is a recipient of an ERC Starting Grant 2013 (European Research Council, Starting Grant 336452-ENIGMO), PDR subsidies (Projet de recherches T0.138.14; Fonds de la Recherche Scientifique, Belgium) and ARC subsidies (Concerted Research Activities-French Community of Belgium convention: 12/17-047) and is supported by the FRS-FNRS via the FRFS-WELBIO under Grant number WELBIO-CR-2012S-02R. LBB and AE are postdoctoral fellows at the FRS-FNRS.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors were responsible for drafting the article and revising it critically for important intellectual content. All authors approved the version to be published.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delzenne, N.M., Cani, P.D., Everard, A. et al. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 58, 2206–2217 (2015). https://doi.org/10.1007/s00125-015-3712-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-015-3712-7