Abstract
Purpose
In between innate and adaptive immunity, the recently identified innate-like mucosal-associated invariant T (MAIT) lymphocytes display specific reactivity to non-streptococcal bacteria. Whether they are involved in bacterial sepsis has not been investigated. We aimed to assess the number and the time course of circulating innate-like T lymphocytes (MAIT, NKT and γδ T cells) in critically ill septic and non-septic patients and to establish correlations with the further development of intensive care unit (ICU)-acquired infections.
Methods
We prospectively enrolled consecutive patients with severe sepsis and septic shock. Controls were critically ill patients with non-septic shock and age-matched healthy subjects. Circulating innate-like lymphocytes were enumerated using a flow cytometry assay at day 1, 4 and 7.
Results
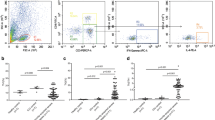
One hundred and fifty six patients (113 severe bacterial infections, 36 non-infected patients and 7 patients with severe viral infections) and 26 healthy subjects were enrolled into the study. Patients with severe bacterial infections displayed an early decrease in MAIT cell count [median 1.3/mm3; interquartile range (0.4–3.2)] as compared to control healthy subjects [31.1/mm3 (12.1–45.2)], but also to non-infected critically ill patients [4.3/mm3 (1.4–13.2)] (P < 0.0001 for all comparisons). In contrast NKT and γδ T cell counts did not differ between patients groups. The multivariate analysis identified non-streptococcal bacterial infection as an independent determinant of decrease in MAIT cell count. Furthermore, the incidence of ICU-acquired infections was higher in patients with persistent MAIT cell depletion.
Conclusions
This large human study provides valuable information about MAIT cells in severe bacterial infections. The persistent depletion of MAIT cells is associated with the further development of ICU-acquired infections.



Similar content being viewed by others
References
Treiner E, Lantz O (2006) CD1d- and MR1-restricted invariant T cells: of mice and men. Curr Opin Immunol 18:519–526
Bendelac A, Bonneville M, Kearney JF (2001) Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol 1:177–186
Bonneville M, Scotet E (2006) Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol 18:539–546
Kawachi I, Maldonado J, Strader C, Gilfillan S (2006) MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol 176:1618–1627
Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T (2006) Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol 7:987–994
Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O (2010) Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11:701–708
Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117:1250–1259
Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O (2011) Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 32:212–218
Grimaldi D (2011) Bacterial sepsis induces a profound depletion in circulating mucosal-associated invariant T (MAIT) lymphocytes (Abstract). Intensive Care Med 37 (Suppl 1):1005
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29:530–538
Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JS, Craven DE (2001) Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis 32:1249–1272
American Thoracic Society, Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Resp Crit Care Med 171:388–416
Anderson JR, Cain KC, Gelber RD (1983) Analysis of survival by tumor response. J Clin Oncol 1:710–719
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491:717–723
Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, Adelmann K, Kang YH, Fergusson JR, Simmonds P, Goulder P, Hansen TH, Fox J, Gunthard HF, Khanna N, Powrie F, Steel A, Gazzard B, Phillips RE, Frater J, Uhlig H, Klenerman P (2013) Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood 121:951–961
Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, Barnes EJ, Thevanayagam J, Bhagwanani A, Leslie A, Oo YH, Kollnberger S, Bowness P, Drognitz O, Adams DH, Blum HE, Thimme R, Klenerman P (2010) Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A 107:3006–3011
Asehnoune K, Roquilly A, Abraham E (2012) Innate immune dysfunction in trauma patients: from pathophysiology to treatment. Anesthesiology 117:411–416
Grimaldi D, Louis S, Pene F, Sirgo G, Rousseau C, Claessens YE, Vimeux L, Cariou A, Mira JP, Hosmalin A, Chiche JD (2011) Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Med 37:1438–1446
Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohe J, Vanhems P, Monneret G (2010) Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med 36:1859–1866
Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D (2009) Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med 37:2746–2752
Guignant C, Venet F, Planel S, Demaret J, Gouel-Cheron A, Nougier C, Friggeri A, Allaouchiche B, Lepape A, Monneret G (2013) Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Med 39:1556–1564
Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Cheron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F (2011) Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 15:R99
Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268
Georgel P, Radosavljevic M, Macquin C, Bahram S (2011) The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol 48:769–775
Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM (2010) Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8:e1000407
Mattner J, Debord KL, Ismail N, Goff RD, Cantu C 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525–529
Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434:520–525
Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M (2006) Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol 7:978–986
Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, Li Y, Imamura M, Kaneko Y, Okawara A, Miyazaki Y, Gomez-Velasco A, Rogers P, Dahesh S, Uchiyama S, Khurana A, Kawahara K, Yesilkaya H, Andrew PW, Wong CH, Kawakami K, Nizet V, Besra GS, Tsuji M, Zajonc DM, Kronenberg M (2011) Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol 12:966–974
Leung B, Harris HW (2011) NKT cells: the culprits of sepsis? J Surg Res 167:87–95
Venet F, Bohe J, Debard AL, Bienvenu J, Lepape A, Monneret G (2005) Both percentage of gammadelta T lymphocytes and CD3 expression are reduced during septic shock. Crit Care Med 33:2836–2840
Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A (2008) Regulatory T cell populations in sepsis and trauma. J Leuk Biol 83:523–535
Deknuydt F, Roquilly A, Cinotti R, Altare F, Asehnoune K (2013) An in vitro model of mycobacterial granuloma to investigate the immune response in brain-injured patients. Crit Care Med 41:245–254
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. New Engl J Med 361:1570–1583
Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Monneret G (2004) Increased percentage of CD4+ CD25+ regulatory T cells during septic shock is due to the decrease of CD4+ CD25− lymphocytes. Crit Care Med 32:2329–2331
Acknowledgments
We are indebted to Professor A. Brezin (Department of Ophtalmology, Cochin Hospital) for including age-matched control subjects, and to Dr. N. Chapuis (Hematology laboratory, Cochin Hospital) and the Cochin Cytometry and Immunobiology Facility for technical help. This work was supported by grants from the European Society of Intensive Care Medicine (ECCRN Clinical Research Award 2010) and from the French Health Department (Inserm/DGOS 2011). D.G. was a recipient of a “Poste d’Accueil CNRS-CEA/APHP.” The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
J.P.M. has been a consultant to MSD, LFB, Astrazeneca, Astellas, Eli Lilly and Brahms and has received research funding from Eli Lilly. O.L. received royalties from Biolegend for the anti-Vα7.2 antibody. F.P. gave a lecture for LFB. J.D.C is the current president of European Society of Intensive Care Medicine. The other authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
A related article can be found at: doi: 10.1007/s00134-013-3141-3.
Take-home message: The innate-like lymphocytes called mucosal-associated invariant T (MAIT) cells are specifically decreased in patients with severe bacterial infections and their changes over time impact the further development of ICU-acquired infections. These results appear specific to MAIT cells among other innate-like T lymphocytes and add MAIT cell depletion to the spectrum of sepsis-induced immunosuppression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grimaldi, D., Le Bourhis, L., Sauneuf, B. et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med 40, 192–201 (2014). https://doi.org/10.1007/s00134-013-3163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-3163-x