Abstract
Purpose
Positron emission tomography (PET) with 11C-5-hydroxytryptophan (5-HTP) as tracer is a promising imaging instrument in the management of patients with neuroendocrine tumours (NETs). However, high radioactivity concentrations in the urinary collecting system sometimes produce image reconstruction artefacts that can make detection of small NETs difficult. As a means to decrease urinary excretion of radioactivity and thereby improve image quality, we examined the effect of pretreatment with carbidopa (CD), a peripheral inhibitor of aromatic amino acid decarboxylase (AADC), which converts 5-HTP to serotonin (5-hydroxytryptamine, 5-HT).
Methods
Six patients with midgut carcinoid metastases were examined with 11C-5-HTP PET before and 1 h after oral administration of 100 or 200 mg of CD.
Results
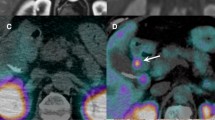
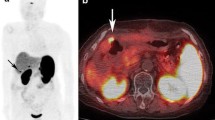
There was a fourfold significant reduction of tracer uptake in the urinary collecting system after CD administration (p=0.0277, n=6), with a mean standard uptake value (SUV) of 155±195 before CD and 39±14 after CD. In tumour lesions there was a significant increase in SUV after CD administration (p<0. 0001, n=18), with a mean SUV of 11±3 before CD and 14±3 after CD. There was no difference between the doses (100 and 200 mg) of CD in this respect. In all patients, image interpretation and tumour detection were markedly improved after CD administration.
Conclusion
We conclude that CD premedication improves 11C-5-HTP PET image quality and facilitates detection of NET lesions. Because of the similarity of metabolic pathways, this method could probably be applied to improve PET imaging using other tracers like 18F-DOPA and 11C-DOPA.


Similar content being viewed by others
References
Pearse AGE. The APUD concept and hormone production. Clin Endocrinol Metab 1980;9:211–22
Norheim I, Oberg K, Theodorsson-Norheim E, Lindgren PG, Lundqvist G, Magnusson A, et al. Malignant carcinoid tumors; an analysis of 103 patients with regard to tumor location, hormone production and survival. Ann Surg 1987;206:115–25
Eriksson B, Arnberg H, Lindgren PG, Lorelius LE, Magnusson A, Lundqvist G, et al. Clinical presentation, biochemical and histopathological findings in 84 patients with neuroendocrine pancreatic tumors. J Intern Med 1990;228:103–13
Kwekkeboom DJ, Krenning EP. Somatostatin receptor scintigraphy in patients with carcinoid tumors. World J Surg 1996;20:157–61
Lebtahi R, Cadiot G, Sarda L, Daou D, Faraggi M, Petegnief Y, et al. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med 1997;38:853–8
Ahlstrom H, Eriksson B, Bergstrom M, Bjurling P, Langstrom B, Oberg K. Pancreatic neuroendocrine tumors—diagnosis with PET. Radiology 1995;195:333–7
Orlefors H, Sundin A, Bjurling P, Bergstrom M, Lilja A, Langstrom B, et al. Positron emission tomography with 5-hydroxytryprophan in neuroendocrine tumors. J Clin Oncol 1998;16:2534–41
Zieger MA, Schawker TH, Norton JA. Use of intraoperative ultrasonography to localize islet cell tumors. World J Surg 1993;17:448–54
Zimmer T, Stolzel U, Bader M, Koppenhagen K, Hamm B, Buhr H, et al. Gut endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localization of insulinomas and gastrinomas. Gut 1996;39:562–8
Orlefors H, Sundin A, Garske U, Juhlin K, Oberg K, Skogseid B, et al. Whole-body 11C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab 2005;90:3392–400
Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and theraphy. J Nucl Med 2000;41:1704–13
Adams S, Baum R, Rink T, Schumm-Drager PM, Usadel KH, Hor G. Limited value of 18F-fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumors. Eur J Nucl Med 1998;25:79–83
Sundin A, Eriksson B, Bergstrom M, Bjurling P, Lindner KJ, Oberg K, et al. Demonstration of 11C-5-hydroxy-L-tryptophan uptake and decarboxylation in carcinoid tumors by specific positioning labeling in PET. Nucl Med Biol 2000;27:33–41
Bergstrom M, Eriksson B, Oberg K, Sundin A, Ahlstrom H, Lindner KJ, et al. In vivo demonstration of enzyme activity in endocrine pancreatic tumors: decarboxylation of 11C-DOPA to 11C-dopamine. J Nucl Med 1996;1:32–7
Rahman MK, Nagatsu T, Kato T. Aromatic L-aminoacid decarboxylase activity in central and peripheral tissues and serum of rats with L-dopa and L-5-hydroxytryptophan as substrates. Biochem Pharmacol 1981;30:645–9
Bjurling P, Antoni G, Watanabe Y, Langstrom B. Enzymatic synthesis of carboxy-11C-labelled L-tyrosine, L-DOPA, L-tryptophan and 5-hydroxytryptophan. Acta Chem Scand 1990;44:178–82
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple time uptake data. J Cereb Blood Flow Metab 1983;3:1–7
Bergstrom M, Lu L, Eriksson B, Marques M, Bjurling P, Andersson Y, et al. Modulation of organ uptake of 11C-labelled 5-hydroxy-tryptophan. Biogenic Amines 1996;12:477–85
Sole MJ, Madapallimattam A, Baines AD. An active pathway for serotonin synthesis by renal proximal tubules. Kidney Int 1986;29:689–94
Itskovitz HD, Werber JL, Sheridan AM, Brewer TF, Stier CT. 5-Hydroxytryptophan and carbidopa in spontaneously hypertensive rats. J Hypertens 1989;7:311–5
Sundin A, Johansson A, Hellman P, Bergstrom M, Ahlstrom H, Jacobsson G.B, et al. PET and parathyroid L-11C-methionine accumulation in hyperparathyroidism. J Nucl Med 1996;37:1766–70
Hoegerle S, Altehoefer C, Ghanem N, Koehler G, Waller CF, Scheruebl H, et al. Whole-body 18F-Dopa PET for detection of gastrointestinal carcinoid tumors. Radiology 2001;220:373–80
Bergstrom M, Eriksson B, Oberg K, Sundin A, Ahlstrom H, Lindner KJ, et al. In vivo demonstration of enzyme activity in endocrine pancreatic tumors: decarboxylation of 11C-DOPA to 11C-dopamine. J Nucl Med 1996;37:32–7
Acknowledgements
This work was supported by the Swedish Cancer Foundation, Lions Cancer Fund and Soderbergs Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Örlefors, H., Sundin, A., Lu, L. et al. Carbidopa pretreatment improves image interpretation and visualisation of carcinoid tumours with 11C-5-hydroxytryptophan positron emission tomography. Eur J Nucl Med Mol Imaging 33, 60–65 (2006). https://doi.org/10.1007/s00259-005-1891-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1891-z