Abstract
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer entity with a high proliferative potential. Uncontrolled cell proliferation is mediated by a number of core signaling pathways. Recently, novel data of PDAC biology suggest that these core signal pathways affect cell proliferation and metabolism simultaneously.
Methods
Here, we reviewed the literature on core metabolic signaling pathways in pancreatic carcinogenesis.
Results
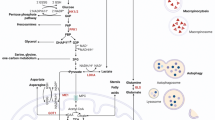
Results obtained from mouse genetics and in vitro experiments have demonstrated the significance of the Kras, p53, c-Myc, and Lkb1 networks in the proliferation of pancreatic epithelial and cancer cells. At the same time, these major pathways also affect energy metabolism by influencing glucose and glutamine utilization. In particular, Kras-mediated metabolic changes seem to be directly involved in carcinogenesis. However, there is a lack of solid evidence on how metabolism and proliferation are connected in pancreatic carcinogenesis.
Conclusion
Understanding early and subtle changes in cellular metabolism of pancreatic epithelial—and specifically of acinar—cells, which accompany or directly influence malignant transformation and uncontrolled proliferation, will be paramount to define novel imaging and other modalities for earlier detection of PDAC.

Similar content being viewed by others
References
DeBerardinis RJ et al (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA: A Cancer Journal for Clinicians 62(1):10–29
Aichler M et al (2011) Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J Pathol doi: 10.1002/path.3017
Vincent A et al (2011) Pancreatic cancer. Lancet 378(9791):607–620
Warburg O, Posener K, Negelein E (1924) Über den Stoffwechsel der Tumoren. Biochem Z 152:319–344
Hunt TK et al (2007) Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal 9(8):1115–1124
Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9(6):425–434
Le A et al (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 107(5):2037–2042
Schneiderhan W et al (2009) CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut 58(10):1391–1398
Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330(6009):1340–1344
Zhou W et al (2012) Proteomic analysis reveals warburg effect and anomalous metabolism of glutamine in pancreatic cancer cells. J Proteome Res 11(2):554–563
Kwon SJ, Lee YJ (2005) Effect of low glutamine/glucose on hypoxia-induced elevation of hypoxia-inducible factor-1alpha in human pancreatic cancer MiaPaCa-2 and human prostatic cancer DU-145 cells. Clin Cancer Res: Off J Am Assoc Cancer Res 11(13):4694–4700
DeBerardinis RJ et al (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104(49):19345–19350
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5):325–337
Frezza C, Pollard PJ, Gottlieb E (2011) Inborn and acquired metabolic defects in cancer. J Mol Med 89(3):213–220
Moskaluk CA, Hruban RH, Kern SE (1997) p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res 57(11):2140–2143
Iacobuzio-Donahue CA et al (2009) DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol: Off J Am Soc Clin Oncol 27(11):1806–1813
Hezel AF et al (2006) Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20(10):1218–1249
Gaglio D et al (2011) Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 7:523
Yun J et al (2009) Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325(5947):1555–1559
Vizan P et al (2005) K-ras codon-specific mutations produce distinctive metabolic phenotypes in NIH3T3 mice [corrected] fibroblasts. Cancer Res 65(13):5512–5515
Chiaradonna F et al (2006) Ras-dependent carbon metabolism and transformation in mouse fibroblasts. Oncogene 25(39):5391–5404
Skoudy A, Hernandez-Munoz I, Navarro P (2011) Pancreatic ductal adenocarcinoma and transcription factors: role of c-Myc. J Gastrointest Cancer 42(2):76–84
Meyer N, Penn LZ (2008) Reflecting on 25 years with MYC. Nat Rev Cancer 8(12):976–990
Schleger C et al (2002) c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol 15(4):462–469
Sears R et al (2000) Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14(19):2501–2514
Wise DR et al (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 105(48):18782–18787
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res: Off J Am Assoc Cancer Res 15(21):6479–6483
Scarpa A et al (1993) Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol 142(5):1534–1543
Pellegata NS et al (1994) K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res 54(6):1556–1560
Oren M (2003) Decision making by p53: life, death and cancer. Cell Death Differ 10(4):431–442
Shen L et al (2012) The fundamental role of the p53 pathway in tumor metabolism and its implication in tumor therapy. Clinical Cancer Res (in press)
Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E (2004) The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 64(7):2627–2633
Bensaad K et al (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126(1):107–120
Mathupala SP, Heese C, Pedersen PL (1997) Glucose catabolism in cancer cells. The type II hexokinase promoter contains functionally active response elements for the tumor suppressor p53. J Biol Chem 272(36):22776–22780
Hu W et al (2010) Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA 107(16):7455–7460
Mehenni H et al (2006) Cancer risks in LKB1 germline mutation carriers. Gut 55(7):984–990
Birnbaum DJ et al (2011) Genome profiling of pancreatic adenocarcinoma. Genes Chromosomes Cancer 50(6):456–465
Alessi DR, Sakamoto K, Bayascas JR (2006) LKB1-dependent signaling pathways. Annu Rev Biochem 75:137–163
Katajisto P et al (2007) The LKB1 tumor suppressor kinase in human disease. Biochim Biophys Acta 1775(1):63–75
Sauer B (1987) Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol 7(6):2087–2096
Sauer B, Henderson N (1988) Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA 85(14):5166–5170
Orban PC, Chui D, Marth JD (1992) Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci USA 89(15):6861–6865
Jonsson J et al (1994) Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371(6498):606–609
Offield MF et al (1996) PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122(3):983–995
Krapp A et al (1998) The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev 12(23):3752–3763
Kawaguchi Y et al (2002) The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 32(1):128–134
Hingorani SR et al (2005) Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7(5):469–483
Tuveson DA et al (2004) Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5(4):375–387
Collins MA et al (2012) Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest 122:639–53
Jones S et al (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321(5897):1801–1806
Morton JP et al (2010) Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA 107(1):246–251
Nakada D, Saunders TL, Morrison SJ (2010) Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468(7324):653–658
Gurumurthy S et al (2010) The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468(7324):659–663
Gan B et al (2010) Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468(7324):701–704
Sato N et al (2001) STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol 159(6):2017–2022
Hezel AF et al (2008) Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol 28(7):2414–2425
Morton JP et al (2010) LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology 139(2):586–597, 597 e1-6
Bonal C et al (2009) Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology 136(1):309–319, e9
Nakhai H et al (2008) Conditional inactivation of Myc impairs development of the exocrine pancreas. Development 135(19):3191–3196
Sandgren EP et al (1991) Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci USA 88(1):93–97
Mazur PK et al (2010) Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA 107(30):13438–13443
Acknowledgments
This study has in part been supported by a grant from the Else-Kröner-Fresenius-Stiftung (to CWM and JK) and from the European Union (EPC-TM-NET, within the FP7 program, to CWM, ME, and JK).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Regel, I., Kong, B., Raulefs, S. et al. Energy metabolism and proliferation in pancreatic carcinogenesis. Langenbecks Arch Surg 397, 507–512 (2012). https://doi.org/10.1007/s00423-012-0933-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-0933-9