Summary
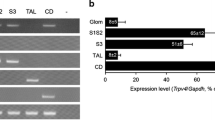
Cellular taurine uptake or release counteracts alterations of cell volume. Na+-coupled taurine transporter TAUT mediates concentrative cellular uptake of taurine. Inhibition of vasopressin secretion by hypotonicity may involve taurine release from glial cells of supraoptic nucleus. We compared renal function of mice lacking TAUT (taut−/−) and wild-type littermates (taut+/+). We observed renal taurine loss and subsequent hypotaurinemia in taut−/− mice. With free access to water, plasma and urine osmolality, urinary flow rate as well as urinary excretion and plasma concentrations of Na+ and K+ were similar in taut−/− and taut+/+ mice, whereas plasma concentrations of urea were enhanced in taut−/− mice. An oral water load (1 ml/16 g body weight) induced a similar diuresis in both genotypes. Repeating the oral water load immediately after normalization of urine flow rate, however, resulted in delayed diuresis and higher urinary vasopressin/creatinine ratios in taut−/− mice. In comparison, the repeated diuretic response to vasopressin V2 receptor blockade was not different between genotypes. Water deprivation for 36 h led to similar antidiuresis and increases of urinary osmolality in both genotypes. Upon free access to water after deprivation, taut−/− mice continued to concentrate urine up to 6 days, while taut+/+ mice rapidly returned to normal urinary osmolality. Urinary vasopressin/creatinine ratios and plasma aldosterone concentrations were not different under basal conditions but were significantly higher in taut−/− mice than in taut+/+ mice at 6 days after water deprivation. In conclusion, taut−/− mice suffer from renal taurine loss and impaired ability to lower urine osmolality and to increase urinary water excretion. The latter defect could reside extrarenally and result from a role of taurine in the suppression of vasopressin release which may be attenuated in taut−/− mice.







Similar content being viewed by others
References
Akizuki N, Uchida S, Sasaki S, Marumo F (2001) Impaired solute accumulation in inner medulla of Clcnk1-/- mice kidney. Am J Physiol Renal Physiol 280:F79–F87
Bedford JJ, Bagnasco SM, Kador PF, Harris HW Jr, Burg MB (1987) Characterization and purification of a mammalian osmoregulatory protein, aldose reductase, induced in renal medullary cells by high extracellular NaCl. J Biol Chem 262:14255–14259
Benyajati S, Bay SM (1994) Basolateral taurine transport system in reptilian renal cells. Am J Physiol 266:F439–F449
Berry GT, Mallee JJ, Kwon HM, Rim JS, Mulla WR, Muenke M, Spinner NB (1995) The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): molecular cloning and localization to chromosome 21. Genomics 25:507–513
Bitoun M, Levillain O, Tappaz M (2001) Gene expression of the taurine transporter and taurine biosynthetic enzymes in rat kidney after antidiuresis and salt loading. Pflugers Arch 442:87–95
Burg MB (1995) Molecular basis of osmotic regulation. Am J Physiol 268:F983–F996
Burnham CE, Buerk B, Schmidt C, Bucuvalas JC (1996) A liver-specific isoform of the betaine/GABA transporter in the rat: cDNA sequence and organ distribution. Biochim Biophys Acta 1284:4–8
Cowley BD Jr, Ferraris JD, Carper D, Burg MB (1990) In vivo osmoregulation of aldose reductase mRNA, protein, and sorbitol in renal medulla. Am J Physiol 258:F154–F161
Ferraris JD, Williams CK, Martin BM, Burg MB, Garcia-Perez A (1994) Cloning, genomic organization, and osmotic response of the aldose reductase gene. Proc Natl Acad Sci USA 91:10742–10746
Garcia-Perez A, Burg MB (1991) Renal medullary organic osmolytes. Physiol Rev 71:1081–1115
Garcia-Perez A, Burg MB (1991) Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membr Biol 119:1–13
Garcia-Perez A, Martin B, Murphy HR, Uchida S, Murer H, Cowley BD Jr, Handler JS, Burg MB (1989) Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J Biol Chem 264:16815–16821
Hammerman MR, Sacktor B, Daughaday WH (1980) myo-Inositol transport in renal brush border vesicles and it inhibition by D-glucose. Am J Physiol 239:F113–F120
Hediger MA, Smith CP, You G, Lee WS, Kanai Y, Shayakul C (1996) Structure, regulation and physiological roles of urea transporters. Kidney Int 49:1615–1623
Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haussinger D (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J 16:231–233
Huang DY, Pfaff I, Serradeil-Le Gal C, Vallon V (2000) Acute renal response to the non-peptide vasopressin V2-receptor antagonist SR 121463B in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol 362:201–207
Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V (2004) Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15:885–891
Hussy N, Deleuze C, Desarmenien MG, Moos FC (2000) Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol 62:113–134
Huxtable RJ, Lippincott SE (1982) Diet and biosynthesis as sources of taurine in the mouse. J Nutr 112:1003–1010
Jones DP, Miller LA, Chesney RW (1993) Polarity of taurine transport in cultured renal epithelial cell lines: LLC-PK1 and MDCK. Am J Physiol 265:F137–F145
Kwon HM (1994) Osmoregulation of Na-coupled organic osmolyte transporters. Ren Physiol Biochem 17:205–207
Kwon HM, Handler JS (1995) Cell volume regulated transporters of compatible osmolytes. Curr Opin Cell Biol 7:465–471
Kwon HM, Yamauchi A, Uchida S, Preston AS, Garcia-Perez A, Burg MB, Handler JS (1992) Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem 267:6297–6301
Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306
Lang PA, Warskulat U, Heller-Stilb B, Huang DY, Grenz A, Myssina S, Duszenko M, Lang F, Haussinger D, Vallon V, Wieder T (2003) Blunted apoptosis of erythrocytes from taurine transporter deficient mice. Cell Physiol Biochem 13:337–346
Martial S, Price SR, Sands JM (1995) Regulation of aldose reductase, sorbitol dehydrogenase, and taurine cotransporter mRNA in rat medulla. J Am Soc Nephrol 5:1971–1978
McKinley MJ, Evered MD, Mathai ML (2000) Renal Na excretion in dehydrated and rehydrated adrenalectomized sheep maintained with aldosterone. Am J Physiol Regul Integr Comp Physiol 279:R17–R24
Moeckel GW, Lai LW, Guder WG, Kwon HM, Lien YH (1997) Kinetics and osmoregulation of Na(+)- and Cl(-)-dependent betaine transporter in rat renal medulla. Am J Physiol 272:F100–F106
Mozaffari MS, Schaffer D (2001) Taurine modulates arginine vasopressin-mediated regulation of renal function. J Cardiovasc Pharmacol 37:742–750
Nakanishi T, Takamitsu Y, Nakahama H, Sugita M (1994) Impairment of renal medullary osmolyte accumulation in potassium-depleted rats. Am J Physiol 267:F139–F145
Nakanishi T, Uyama O, Nakahama H, Takamitsu Y, Sugita M (1993) Determinants of relative amounts of medullary organic osmolytes: effects of NaCl and urea differ. Am J Physiol 264:F472–F479
Nakanishi T, Uyama O, Sugita M (1991) Osmotically regulated taurine content in rat renal inner medulla. Am J Physiol 261:F957–F962
Nakanishi T, Uyama O, Yamada T, Sugita M (1992) Sustained metabolic alkalosis associated with development of the milk-alkali syndrome. Nephron 60:251
Nakanishi T, Yamauchi A, Sugita M, Takamitsu Y (1996) Aldose reductase and myo-inositol transporter mRNA are independently regulated in rat renal medulla. J Am Soc Nephrol 7:283–289
Rauchman MI, Nigam SK, Delpire E, Gullans SR (1993) An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol 265:F416–F424
Shayakul C, Hediger MA (2004) The SLC14. gene family of urea transporters. Pflugers Arch 447:603–609
Sturman JA, Hepner GW, Hofmann AF, Thomas PJ (1975) Metabolism of [35S]taurine in man. J Nutr 105:1206–1214
Takenaka M, Bagnasco SM, Preston AS, Uchida S, Yamauchi A, Kwon HM, Handler JS (1995) The canine betaine gamma-amino-n-butyric acid transporter gene: diverse mRNA isoforms are regulated by hypertonicity and are expressed in a tissue-specific manner. Proc Natl Acad Sci USA 92:1072–1076
Thrasher TN, Wade CE, Keil LC, Ramsay DJ (1984) Sodium balance and aldosterone during dehydration and rehydration in the dog. Am J Physiol 247:R76–R83
Uchida S, Kwon HM, Yamauchi A, Preston AS, Marumo F, Handler JS (1992) Molecular cloning of the cDNA for an MDCK cell Na(+)- and Cl(-)-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci USA 89:8230–8234
Vallon V (2003) In vivo studies of the genetically modified mouse kidney. Nephron Physiol 94:1–5
Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D (2002) Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest 110:1263–1268
Yamauchi A, Kwon HM, Uchida S, Preston AS, Handler JS (1991) Myo-inositol and betaine transporters regulated by tonicity are basolateral in MDCK cells. Am J Physiol 261:F197–F202
Yamauchi A, Sugiura T, Ito T, Miyai A, Horio M, Imai E, Kamada T (1996) Na+/myo-inositol transport is regulated by basolateral tonicity in Madin-Darby canine kidney cells. J Clin Invest 97:263–267
Yamauchi A, Uchida S, Kwon HM, Preston AS, Robey RB, Garcia-Perez A, Burg MB, Handler JS (1992) Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem 267:649–652
Yancey PH, Burg MB (1989) Distribution of major organic osmolytes in rabbit kidneys in diuresis and antidiuresis. Am J Physiol 257:F602–F607
Yao L, Huang DY, Pfaff IL, Nie X, Leitges M, Vallon V (2004) Evidence for a role of protein kinase C-alpha in urine concentration. Am J Physiol Renal Physiol 287:F299–F304
Acknowledgements
This work was supported by grants from DFG, the Department of Veterans Affairs, the National Institutes of Health (DK56248, DK28602) and BMBF (F.L., V.V., D.H.). SR121463 was kindly provided by C. Serradeil-Le Gal, Sanofi-Synthelabo, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, D.Y., Boini, K.M., Lang, P.A. et al. Impaired ability to increase water excretion in mice lacking the taurine transporter gene TAUT. Pflugers Arch - Eur J Physiol 451, 668–677 (2006). https://doi.org/10.1007/s00424-005-1499-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-005-1499-y