Abstract
Background
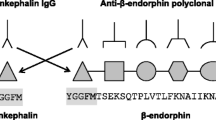
Endogenous opioids, including enkephalins, are fundamental regulators of pain. In inflammatory conditions, the local release of opioids by leukocytes at the inflammatory site inhibits nociceptor firing, thereby inducing analgesia. Accordingly, in chronic intestinal Th1/Th17-associated inflammation, enkephalins released by colitogenic CD4+ T lymphocytes relieve inflammation-induced visceral pain. The present study aims to investigate whether mucosal T-cell-derived enkephalins also exhibit a potent anti-inflammatory activity as described for exogenous opioid drugs in Th1/Th17-associated colitis.
Methods
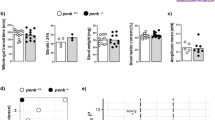
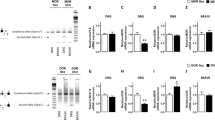
The anti-inflammatory effects of endogenous opioids were investigated in both Th1/Th17-associated (transfer of CD4+CD45RBhigh T lymphocytes) and Th2-associated (oxazolone) colitis models in mice. Inflammation-induced colonic damage and CD4+ T cell subsets were compared in mice treated or not treated with naloxone methiodide, a peripheral antagonist of opioid receptors. The anti-inflammatory activity of T-cell-derived enkephalins was further estimated by comparison of colitis severity in immunodeficient mice into which naïve CD4+CD45RBhigh T lymphocytes originating from wild-type or enkephalin-knockout mice had been transferred.
Results
Peripheral opioid receptor blockade increases the severity of Th1/Th17-induced colitis and attenuates Th2 oxazolone colitis. The opposite effects of naloxone methiodide treatment in these two models of intestinal inflammation are dependent on the potency of endogenous opioids to promote a Th2-type immune response. Accordingly, the transfer of enkephalin-deficient CD4+CD45RBhigh T lymphocytes into immunodeficient mice exacerbates inflammation-induced colonic injury.
Conclusions
Endogenous opioids, including T-cell-derived enkephalins, promote a Th2-type immune response, which, depending on the context, may either attenuate (Th1/Th17-associated) or aggravate (Th2-associated) intestinal inflammation.






Similar content being viewed by others
References
Stein C. Opioids, sensory systems and chronic pain. Eur J Pharmacol. 2013;716:179–87.
Boue J, Blanpied C, Brousset P, et al. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J Immunol. 2011;186:5078–84.
Basso L, Boue J, Mahiddine K, et al. Endogenous analgesia mediated by CD4+ T lymphocytes is dependent on enkephalins in mice. J Neuroinflammation 2016;13:132.
Boue J, Basso L, Cenac N, et al. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4+ T cells in mice. Gastroenterology. 2014;146:166–75.
Basso L, Bourreille A, Dietrich G. Intestinal inflammation and pain management. Curr Opin Pharmacol. 2015;25:50–5.
Boue J, Blanpied C, Djata-Cabral M, et al. Immune conditions associated with CD4+ T effector-induced opioid release and analgesia. Pain. 2012;153:485–93.
Stein C, Kuchler S. Non-analgesic effects of opioids: peripheral opioid effects on inflammation and wound healing. Curr Pharm Des. 2012;18:6053–69.
Anselmi L, Huynh J, Duraffourd C, et al. Activation of mu opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol Motil. 2015;27:509–23.
Goldsmith JR, Uronis JM, Jobin C. Mu opioid signaling protects against acute murine intestinal injury in a manner involving Stat3 signaling. Am J Pathol. 2011;179:673–83.
Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu-opioid receptor support its use in the treatment of colon inflammation. J Clin Investig. 2003;111:1329–38.
Sobczak M, Salaga M, Storr MA, et al. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol. 2014;49:24–45.
Benard A, Boue J, Chapey E, et al. Delta opioid receptors mediate chemotaxis in bone marrow-derived dendritic cells. J Neuroimmunol. 2008;197:21–8.
Jaume M, Laffont S, Chapey E, et al. Opioid receptor blockade increases the number of lymphocytes without altering T cell response in draining lymph nodes in vivo. J Neuroimmunol. 2007;188:95–102.
Roy S, Wang J, Charboneau R, et al. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J Immunol. 2005;175:6361–7.
Sacerdote P, Manfredi B, Gaspani L, et al. The opioid antagonist naloxone induces a shift from type 2 to type 1 cytokine pattern in BALB/cJ mice. Blood. 2000;95:2031–6.
Cenac N, Cellars L, Steinhoff M, et al. Proteinase-activated receptor-1 is an anti-inflammatory signal for colitis mediated by a type 2 immune response. Inflamm Bowel Dis. 2005;11:792–8.
Noguchi K, Gel YR, Brunner E, et al. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw 2012; 50(12). doi:10.18637/jss.v050.i12
Valdez-Morales E, Guerrero-Alba R, Ochoa-Cortes F, et al. Release of endogenous opioids during a chronic IBD model suppresses the excitability of colonic DRG neurons. Neurogastroenterol Motil. 2013;25:39–46.
Owczarek D, Cibor D, Mach T, et al. Met-enkephalins in patients with inflammatory bowel diseases. Adv Med Sci. 2011;56:158–64.
Baddack-Werncke U, Busch-Dienstfertig M, Gonzalez-Rodriguez S, et al. Cytotoxic T cells modulate inflammation and endogenous opioid analgesia in chronic arthritis. J Neuroinflammation. 2017;14:30.
Basso L, Boue J, Bourreille A, et al. Endogenous regulation of inflammatory pain by T-cell-derived opioids: when friend turns to foe. Inflamm Bowel Dis. 2014;20:1870–7.
Zimmermann J, Kuhl AA, Weber M, et al. T-bet expression by Th cells promotes type 1 inflammation but is dispensable for colitis. Mucosal Immunol. 2016;9:1487–99.
Wang J, Barke RA, Charboneau R, et al. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–34.
Nguyen K, Miller BC. CD28 costimulation induces delta opioid receptor expression during anti-CD3 activation of T cells. J Immunol. 2002;168:4440–5.
Benard A, Cavailles P, Boue J, et al. µ-Opioid receptor is induced by IL-13 within lymph nodes from patients with Sezary syndrome. J Investig Dermatol. 2010;130:1337–44.
Borner C, Woltje M, Hollt V, et al. STAT6 transcription factor binding sites with mismatches within the canonical 5′-TTC…GAA-3′ motif involved in regulation of delta- and mu-opioid receptors. J Neurochem. 2004;91:1493–500.
Kraus J, Borner C, Giannini E, et al. Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J Biol Chem. 2001;276:43901–8.
Griseri T, Arnold IC, Pearson C, et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity. 2015;43:187–99.
Sobczak M, Pilarczyk A, Jonakowski M, et al. Anti-inflammatory and antinociceptive action of the dimeric enkephalin peptide biphalin in the mouse model of colitis: new potential treatment of abdominal pain associated with inflammatory bowel diseases. Peptides. 2014;60:102–6.
Goldsmith JR, Perez-Chanona E, Yadav PN, et al. Intestinal epithelial cell-derived mu-opioid signaling protects against ischemia reperfusion injury through PI3 K signaling. Am J Pathol. 2013;182:776–85.
Borner C, Kraus J. Inhibition of NF-κB by opioids in T cells. J Immunol. 2013;191:4640–7.
Borner C, Warnick B, Smida M, et al. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol. 2009;183:882–9.
Roy S, Wang J, Gupta S, et al. Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet. J Neuroimmunol. 2004;147:78–81.
Wang J, Barke RA, Charboneau R, et al. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278:37622–31.
Acknowledgements
The authors thank the ANEXPLO (UMR 006) animal care facility (Y. Barreira and S. Appolinaire), Aninfimip, an EquipEx (Equipement d’Excellence) supported by the French government through the Investments for the Future program (ANR-11-EQPX-0003), and the U1043 flow cytometry facility (F. L’Faqihi-Olive and V. Duplan-Eche). This work was supported by the Institut National de la Santé et de la Recherche Médicale, Université Paul Sabatier, Toulouse III, and the Association François Aupetit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Basso, L., Garnier, L., Bessac, A. et al. T-lymphocyte-derived enkephalins reduce Th1/Th17 colitis and associated pain in mice. J Gastroenterol 53, 215–226 (2018). https://doi.org/10.1007/s00535-017-1341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1341-2