Abstract
Purpose
Multiple cell types of the tumour microenvironment, including macrophages, contribute to the response to cancer therapy. The anti-resorptive agent zoledronic acid (ZOL) has anti–tumour effects in vitro and in vivo, but it is not known to what extent macrophages are affected by this agent. We have therefore investigated the effects of ZOL on macrophages using a combination of in vitro and in vivo models.
Methods
J774 macrophages were treated with ZOL in vitro, alone and in combination with doxorubicin (DOX), and the levels of apoptosis and necrosis determined. Uptake of zoledronic acid was assessed by detection of unprenylated Rap1a in J774 macrophages in vitro, in peritoneal macrophages and in macrophage populations isolated from subcutaneously implanted breast cancer xenografts following ZOL treatment in vivo.
Results
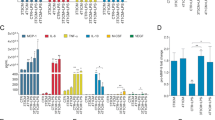
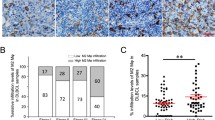
Exposure of J774 macrophages to 5 μM ZOL for 24 h caused a significant increase in the levels of uRap1A, and higher doses/longer exposure induced apoptotic cell death. DOX (10 nM/24 h) and ZOL (10 μM/4 h) given in sequence induced significantly increased levels of apoptotic cell death compared to single agents. Peritoneal macrophages and macrophage populations isolated from breast tumour xenografts had detectable levels of uRap1A 24 h following a single, clinically achievable dose of 100 μg/kg ZOL in vivo.
Conclusion
We demonstrate that macrophages are sensitive to sequential administration of DOX and ZOL, and that both peritoneal and breast tumour associated macrophages rapidly take up ZOL in vivo. Our data support that macrophages may contribute to the anti-tumour effect of ZOL.








Similar content being viewed by others
References
R. Coleman, M. Gnant, G. Morgan, P. Clezardin, Effects of bone-targeted agents on cancer progression and mortality. J. Natl. Cancer Inst. 104(14), 1059–1067 (2012)
M.J. Rogers, K.M. Chilton, F.P. Coxon, J. Lawry, M.O. Smith, S. Suri et al., Bisphosphonates induce apoptosis in mouse macrophage-like cells in vitro by a nitric oxide-independent mechanism. J. Bone Miner. Res. 11(10), 1482–1491 (1996)
M.G. Cecchini, R. Felix, H. Fleisch, P.H. Cooper, Effect of bisphosphonates on proliferation and viability of mouse bone marrow-derived macrophages. J. Bone Miner. Res. 2(2), 135–142 (1987)
M.F. Moreau, C. Guillet, P. Massin, S. Chevalier, H. Gascan, M.F. Baslé et al., Comparative effects of five bisphosphonates on apoptosis of macrophage cells in vitro. Biochem. Pharmacol. 73(5), 718–723 (2007)
E. Giraudo, M. Inoue, D. Hanahan, An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J. Clin. Invest. 114(5), 623–633 (2004)
S.P. Luckman, F.P. Coxon, F.H. Ebetino, R.G.G. Russell, M.J. Rogers, Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J. Bone Miner. Res. 13(11), 1668–1678 (1998)
J.C. Frith, M.J. Rogers, Antagonistic effects of different classes of bisphosphonates in osteoclasts and macrophages in vitro. J. Bone Miner. Res. 18(2), 204–212 (2003)
H. Mönkkönen, P.D. Ottewell, J. Kuokkanen, J. Mönkkönen, S. Auriola, I. Holen, Zoledronic acid-indcuced IPP/ApppI production in vivo. Life Sci. 81(13), 1066–1070 (2007)
T.L. Rogers, I. Holen, Tumour macrophages as potential targets of bisphosphonates. J. Transl. Med. 9(1), 177 (2011)
J.A. Joyce, J.W. Pollard, Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9(4), 239–252 (2009)
S.B. Coffelt, R. Hughes, C.E. Lewis, Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim. Biophys. Acta 1796(1), 11–18 (2009)
S.B. Coffelt, C.E. Lewis, L. Naldini, J.M. Brown, N. Ferrara, M. De Palma, Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am. J. Pathol. 176(4), 1564–1576 (2010)
R.D. Leek, A.L. Harris, Tumor-associated macrophages in breast cancer. J. Mammary Gland Biol. Neoplasia 7(2), 177–189 (2002)
M. Marra, G. Salzano, C. Leonetti, M. Porru, R. Franco, S. Zappavigna, G. Liguori, G. Botti, P. Chieffi, M. Lamberti, G. Vitale, A. Abbruzzese, M.I. La Rotonda, G. De Rosa, M. Caraglia, New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol. Adv. 30(1), 302–309 (2012)
M. Marra, G. Salzano, C. Leonetti, P. Tassone, M. Scarsella, S. Zappavigna, T. Calimeri, R. Franco, G. Liguori, G. Cigliana, R. Ascani, M.I. La Rotonda, A. Abbruzzese, P. Tagliaferri, M. Caraglia, G. De Rosa, Nanotechnologies to use bisphosphonates as potent anticancer agents: the effects of zoledronic acid encapsulated into liposomes. Nanomedicine 7(6), 955–964 (2011)
M. Coscia, E. Quaglino, M. Iezzi, C. Curcio, F. Pantaleoni, C. Riganti, I. Holen, H. Mönkkönen, M. Boccadoro, G. Forni, P. Musiani, A. Bosia, F. Cavallo, M. Massaia, Zoledronic acid repolarizes tumor-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J. Cell. Mol. Med. 4(12), 2803–2815 (2009)
P.D. Ottewell, D.V. Lefley, S.S. Cross, C.A. Evans, R.E. Coleman, I. Holen, Sustained inhibition of tumour growth and prolonged survival following sequential administration of doxorubicin and zoledronic acid in a breast cancer model. Int. J. Cancer 126(2), 522–532 (2010)
P.D. Ottewell, H. Mönkkönen, M. Jones, D.V. Lefley, R.E. Coleman, I. Holen, Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J. Natl. Cancer Inst. 100(16), 1167–1178 (2008)
P.D. Ottewell, H.K. Brown, M. Jones, T.L. Rogers, S.S. Cross, N.J. Brown, R.E. Coleman, I. Holen, Combination therapy inhibits development and progression of mammary tumours in immunocompetent mice. Breast Cancer Res. Treat. 133(2), 523–536 (2012)
H.L. Neville-Webbe, A. Rostami-Hodjegan, C.A. Evans, R.E. Coleman, I. Holen, Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int. J. Cancer 113(3), 364–371 (2005)
R.D. Clyburn, P. Reid, C.A. Evans, D.V. Lefley, I. Holen, Increased anti-tumour effects of doxorubicin and zoledronic acid in prostate cancer cells in vitro – supporting the benefits of combination therapy. Chemother. Pharmacol. 65(5), 969–978 (2009)
I. Holen, R.E. Coleman, Anti-tumour activity of bisphosphonates in preclinical models of breast cancer. Breast Cancer Res. 12(6), 214 (2010)
S.L. Chinault, J.L. Prior, K.M. Kaltenbronn, A. Penly, K.N. Weilbaecher, D. Piwnica-Worms, K.J. Blumer, Breast cancer cell targeting by prenylation inhibitors elucidated in living animals with a bioluminescence reporter. Clin. Cancer Res. 18(15), 4136–4144 (2012)
C. Melani, S. Sangaletti, F.M. Barazzetta, Z. Werb, M.P. Colombo, Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 67(23), 11438–11446 (2007)
W. Zhang, X.D. Zhu, H.C. Sun, Y.Q. Xiong, P.Y. Zhuang, H.X. Xu, L.Q. Kong, L. Wang, W.Z. Wu, Z.Y. Tang, Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin. Cancer Res. 16(13), 3420–3430 (2010)
J.D. Veltman, M.E. Lambers, M. van Nimwegen, R.W. Hendriks, H.C. Hoogsteden, J.P. Hegmans, J.G. Aerts, Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br. J. Cancer 103(5), 629–641 (2010)
R.E. Coleman, H. Marshall, D. Cameron, D. Dodwell, R. Burkinshaw, M. Keane, M. Gil, S.J. Houston, R.J. Grieve, P.J. Barrett-Lee, D. Ritchie, J. Pugh, C. Gaunt, U. Rea, J. Peterson, C. Davies, V. Hiley, W. Gregory, R. Bell, AZURE Investigators, Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 365(15), 1396–1405 (2011)
Acknowledgments
This study was supported by a grant from Weston Park Hospital Cancer Charity, Sheffield, UK. Expert technical support was provided by Mrs Alyson Evans and Ms Sue Newton.
Conflict of interest statement
The authors have no conflicting interests to declare in relation to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rogers, T.L., Wind, N., Hughes, R. et al. Macrophages as potential targets for zoledronic acid outside the skeleton—evidence from in vitro and in vivo models. Cell Oncol. 36, 505–514 (2013). https://doi.org/10.1007/s13402-013-0156-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-013-0156-2