Abstract
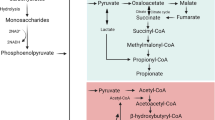
The disease process of ulcerative colitis (UC) is associated with a block in β-oxidation of short chain fatty acid in colonic epithelial cells which can be reproduced by exposure of cells to sulfides. The aim of the current work was to assess the level in the β-oxidation pathway at which sulfides might be inhibitory in human colonocytes. Isolated human colonocytes from cases without colitis (n = 12) were exposed to sulfide (1.5 mM) in the presence or absence of exogenous CoA and ATP. Short chain acyl-CoA esters were measured by a high performance liquid chromatographic assay. 14CO2 generation was measured from [1-14C]butyrate and [6-14C]glucose. 14CO2 from butyrate was significantly reduced (p < 0.001) by sulfide. When colonocytes were incubated with hydrogen sulfide in the presence of CoA and ATP, butyryl-CoA concentration was increased (p < 0.01), while crotonyl-CoA (p < 0.01) and acetyl-CoA (p < 0.01) concentrations were decreased. These results show that sulfides inhibit short chain acyl-CoA dehydrogenase. As oxidation of n-butyrate governs the epithelial barrier function of colonocytes the functional activity of short chain acyl-CoA dehydrogenase may be critical in maintaining colonic mucosal integrity. Maintaining the functional activity of dehydrogenases could be an important determinant in the expression of ulcerative colitis.
Similar content being viewed by others
References
Henning S, Hird FJR: Ketogenesis from butyrate and acetate by the caecum and colon of rabbits. Biochem J 130: 785–790, 1972
Roediger WEW: Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. GUT 21: 793–798, 1980
Ardawi MSM, Newsholme EA: Fuel utilization in colonocytes of the rat. Biochem J 231: 713–719, 1985
Roediger WEW: The colonic epithelium in ulcerative colitis: An energydeficiency disease? Lancet 2: 712–715, 1980
Chapman MAS, Grahn MF, Boyle MA, Hutton M, Rogers J, Williams NS: Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. GUT 35: 73–76, 1994
Roediger WEW, Lawson MJ, Kwok V, Kerr Grant A, Pannall PR: Colonic bicarbonate output as a test of disease activity in ulcerative colitis. J Clin Pathol 37: 704–707, 1984
Den Hond E, Hiele M, Ghoos Y, Rutgeerts P: In vivo colonic butyrate metabolism in extensive ulcerative colitis. Gastroenterology 110: A797, 1996
Roediger WEW, Duncan A, Kapaniris O, Millard S: Reducing sulfur compounds of the colon impair colonocyte nutrition: Implications for ulcerative colitis. Gastroenterology 104: 802–809, 1993
Levine, J, Ellis CJ, Furne JK, Springfield JR, Levitt MD: Sulfate reducing bacteria and ulcerative colitis. Gastroenterology 110: A959, 1996
Pitcher MCL, Cummings JH: Hydrogen sulfide: A bacterial toxin in ulcerative colitis? GUT 39: 1–4, 1996
Roediger WEW, Duncan A, Kapaniris O, Millard S: Sulfide impairment of substrate oxidation in rat colonocytes: A biochemical basis for ulcerative colitis? Clin Sci 85: 623–627, 1993
Lehninger AL: Biochemistry: The Molecular Basis of Cell Structure and Function. Worth Publishers, New York, 1970, pp 365–393
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schönekess BD: Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213: 263–276, 1994
Kunau WH, Dommes V, Schulz H: β-oxidation of fatty acids in mitochondria, peroxisomes and bacteria: A century of continued progress. Prog Lipid Res 34: 267–342, 1995
Schulz H: Oxidation of fatty acids. In: DE Vance, JE Vance (eds). Biochemistry of Lipids, Lipoproteins and Membranes. Elsevier BV, 1996, pp 75–99
MacFarlane GT, Gibson GR, Cummings JH: Comparison of fermentation reactions in different regions of the human colon. Appl Bacteriol J 72: 57–64, 1992
Stanley KK, Tubbs PK: The role of intermediates in mitochondrial fatty acid oxidation. Biochem J 150: 77–88, 1975
Roediger WEW, Truelove SC: Method of preparing isolated colonic epithelial cells (colonocytes) for metabolic studies. GUT 20: 484–488, 1979
Demoz A, Garras A, Asiedu DK, Netteland B, Berge RK: Rapid method for the separation and detection of tissue short chain coenzyme A esters by reversed-phase high-performance liquid chromatography. J Chromat B 667: 148–152, 1995
Corkey BE: Analysis of acyl-coenzyme A esters in biological samples. Meth Enzymol 166: 55–70, 1988
De Buysere MS, Olson MS: The analysis of acyl-coenzyme A derivatives by reverse-phase high-performance liquid chromatography. Anal Biochem 133: 373–379, 1983
Bergmeyer HU, Bergmeyer J, Grassl M: Methods of enzymic analysis. 3rd edn. Weinhein: Verlag Chemie, 1984, Vol VI: pp 582–588, Vol VIII: pp 61–69
Corkey BE, Brandt M, Williams RJ, Williamson JR: Assay of shortchain acyl coenzyme A intermediates in tissue extracts by high-pressure liquid chromatography. Anal Biochem 118: 30–41, 1981
King MT, Reiss PD, Cornell NW: Determination of short-chain coenzyme A compounds by reversed-phase high-performance liquid chromatography. Meth Enzymol 166: 70–79, 1988
Watmough NJ, Turnbull DM, Sherratt HSA, Bartlett K: Measurement of the acyl-CoA intermediates of β-oxidation by h.p.l.c. with on line radiochemical and photodiode-array detection. Biochem J 262: 261–269, 1989
Hovik R, Brodal B, Bartlett K, Osmundsen H: Metabolism of acetyl-CoA by isolated peroxisomal fractions: Formation of acetate and acetoacetyl-CoA. J Lipid Res 32: 993–999, 1991
Bartlett K, Eaton S: Intermediates of mitochondrial β-oxidation. Biochem Soc Trans 22: 432–436, 1994
Radcliffe BC, Nance SH, Roediger WEW: The isolation of coupled mitochondria from rat colonic mucosa. Proc Aust Soc Med Res 19: 24, 1986
Bartlett K, Hovik R, Eaton S, Watmough NJ, Osmundsen H: Intermediates of peroxisomal β-oxidation. Biochem J 270: 175–180, 1990
Ash R, Baird GD: Activation of volatile fatty acids in bovine liver and rumen epithelium. Evidence for control by autoregulation. Biochem J 136: 311–319, 1973
Scaife JR, Tichivangana JZ: Short chain acyl-CoA synthesis in bovine rumen epithelium. Biochim Biophys Acta 619: 445–450, 1980
Bremer J, Wojtczak AB: Factors controlling the rate of fatty acid β-oxidation in rat liver mitochondria. Biochim Biophys Acta 280: 515–530, 1972
Lumeng L, Bremer J, Davis J: Suppression of the mitochondrial oxidation of (–) palmitylcarnitine by the malate-aspartate and x glycerophosphate shuttles. Biol Chem J 251: 277–284, 1976
Latipää PM, Kärki TT, Hiltunen JK, Hassinen IE: Regulation of palmityl carnitine oxidation in isolated rat liver mitochondria. Role of the redox state of NAD(H). Biochim Biophys Acta 875: 293–360, 1986
Eaton S, Turnbull DM,. Bartlett K: Redox control of β-oxidation in rat liver mitochondria. Eur Biochem J 220: 671–681, 1994
Corkey BE, Deeney JT: Acyl-CoA regulation of metabolism and signal transduction. In: K Tanaka, PM Coates (eds). Fatty Acid Oxidation. Clinical, Biochemical and Molecular Aspects. Alan R. Liss, New York, 1990, pp 217–232
Kler RS, Jackson S, Bartlett K: Quantitation of acyl-CoA and acylcarnitine esters accumulated during abnormal mitochondrial fatty acid oxidation. J Biol Chem 266: 22932–22938, 1991
Corkey BE, Hale DE, Glennon MC, Kelley RI, Coates PM, Kilpatrick L, Stanley CA: Relationship between unusual hepatic acyl coenzyme A profiles and the pathogenesis of Reye syndrome. J Clin Invest 82: 782–788, 1988
Dieuaide M, Couee I, Pradet A, Raymond P: Effects of glucose starvation on the oxidation of fatty acids by maize root tip mitochondria and peroxisomes: Evidence for mitochondrial fatty acid β-oxidation and acyl-CoA dehydrogenase activity in a higher plant. Biochem J 296: 199–207, 1993
Keilin D: The History of Cell Respiration and Cytochrome. Cambridge University Press, 1966
Khan AA, Schuler MM, Prior MG, Yong PS, Coppock RW, Florence ZZ, Lillie LE: Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol App Pharmacol 103: 482–490, 1990
Roediger WEW: The place of short chain fatty acids in colonocyte metabolism in health and ulcerative colitis: The impaired colonocyte barrier. In: JH Cummings, JL Rombeau, T Sakata (eds). Physiological and Clinical Aspects of Short Chain Fatty Acids. Cambridge University Press, 1995, pp 337–351
Finnie IA, Dwarkanath AD, Taylor BA, Rhodes JM: Colonic mucin synthesis is increased by sodium butyrate. GUT 36: 93–99, 1995
Roediger WEW, Kapaniris O, Millard S: Lipogenesis from n-butyrate in colonocytes. Action of reducing agent and 5-aminosalicylic acid with relevance to ulcerative colitis. Mol Cell Biochem 118: 113–118, 1992
Frankel W, Lew J, Su B, Klurfeld D, Einhorn E, MacDermott RP, Rombeau J: Butyrate increases colonocyte protein synthesis in ulcerative colitis. Surg Res J 57: 210–214, 1994
Sakata T, Yajima T: Influence of short-chain fatty acids on the epithelial cell diversion of the digestive tract. Quart Exp Physiol J 69: 639–648, 1984
Finnie IA, Taylor BA, Rhodes JM: Ileal and colonic epithelial metabolism in quiescent ulcerative colitis: increased glutamine metabolism in distal colon but no defect in butyrate metabolism. GUT 34: 1552–1558, 1993
Clausen MR, Mortensen PB: Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. GUT 37: 684–689, 1995
Duffy MM, Regan MC, Harrington MG, O’Connell PR: Colonic substrate in Crohn’s disease. Gastroenterology 110: A433, 1996
Ireland A, Jewell DP: 5-aminosalicylic acid (5-ASA) has no effect on butyrate metabolism in human colonic epithelial cells. Gastroenterology 98: A176, 1990
Roediger WEW, Radcliffe BC, Deakin EJ, Nance SH: Specific metabolic effect of sodium nitrite on fat metabolism by mucosal cells of the colon. Dig Dis Sci 31: 535–539, 1986
Roediger WEW, Nance S: Selective reduction of fatty acid oxidation in colonocytes: Correlation with ulcerative colitis. Lipids 25: 646–652, 1990
Allan ES, Winter S, Light AM, Allan A: Mucosal enzyme activity for butyrate oxidation; no defect in patients with ulcerative colitis. GUT 38: 886–893, 1996
Sumegi B, Porpaczy, Alkonyi I: Kinetic advantage of the interaction between the fatty acid β-oxidation enzymes and the complexes of the respiratory chain. Biochim Biophys Acta 1081: 121–128, 1991
Osmundsen H, Bartlett K, Pourfarzam M, Eaton S, Sleboda J: Substrate channelling in β-oxidation: Myth or reality. In: L Agius, HSA Sherratt (eds). Channelling in Intermediary Metabolism. Portland Press, London, 1997, pp 293–313
Gustafson WG, Feinberg BA, MacFarland JT: Energetics of β-oxidation. Biol Chem J 261: 7733–7741, 1986
Engel PC: Acyl-coenzyme A dehydrogenases. In: F Muller (ed). Chemistry and Biochemistry of Flavo-enzymes, Vol III. CRC Press, Boca Raton, USA, 1990, pp 597–655
Willard J, Vicanek C, Battaile KP, Veldhoven PP, Fauq AH, Rozen R, Vockley J: Cloning of a cDNA for short/branched chain acyl-coenzyme A dehydrogenase from rat and characterization of its tissue expression and substrate specifically. Arch Biochem Biophys 331: 127–133, 1996
Williamson G, Engel PC, Mizzer JP, Thorpe C, Massey V: Evidence that the greening ligand in native butyryl-CoA dehydrogenase is a CoA-persulfide. J Biol Chem 257: 4314–4320, 1982
Shaw L, Engel PC: CoA-persulfide: A possible in vivo inhibitor of mammalian short-chain acyl-CoA dehydrogenase. Biochim Biophys Acta 919: 171–174, 1987
Shaw L, Engel PC: The purification and properties of ox liver short-chain acyl-CoA dehydrogenase. Biochem J 218: 511–520, 1984
Engel P: Butyryl-CoA dehydrogenase from Megasphaera elsdenii. Meth Enzymol 71: 359–366, 1981
Fromenty B, Pessayre D: Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther 67: 101–154, 1995
Babidge WJ, Millard SH, WEW Roediger: Thiol methyltransferase activity in colonocytes and erythrocyte membranes Clin Pathol 48: 641–644, 1995
Ramakrishna BS, Roberts-Thomson IC, Pannall PR, WEW Roediger: Impaired sulphation of phenol by the colonic mucosa in quiescent and active ulcerative colitis. GUT 32: 46–49, 1991
Roediger WEW, Babidge W, Millard S: Methionine derivatives diminish sulfide damage to colonocytes – implication for ulcerative colitis. GUT 39: 77–81, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Babidge, W., Millard, S. & Roediger, W. Sulfides impair short chain fatty acid β-oxidation at acyl-CoA dehydrogenase level in colonocytes: Implications for ulcerative colitis. Mol Cell Biochem 181, 117–124 (1998). https://doi.org/10.1023/A:1006838231432
Issue Date:
DOI: https://doi.org/10.1023/A:1006838231432